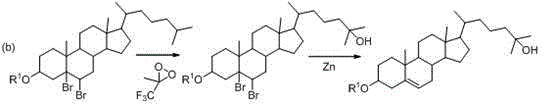
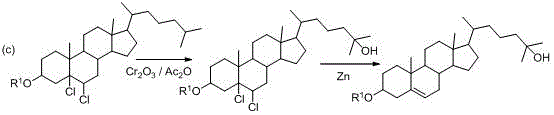
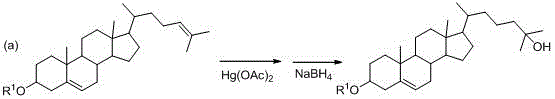A kind of synthetic method of 25-hydroxycholesterol
A technology of hydroxycholesterol and its synthesis method, which is applied in the fields of steroids and organic chemistry, and can solve problems such as difficult industrialization, Hg residue, and difficult operation, and achieve convenient operation and post-processing, mild reaction conditions, and post-processing simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] At room temperature (20-25°C), dissolve 4.27g (10mmol) 24-dehydrocholesterol acetate in 20mL toluene, add 6.01g (100mmol) acetic acid, 49.2mg (0.1mmol) scandium trifluoromethanesulfonate (Sc(OTf) 3 ), heat up to 50°C, stir for 5 hours, after TLC traces the reaction is complete, add 10mL of water layer to remove the water phase, add sodium hydroxide aqueous solution to the organic phase, keep the system pH ≥ 12, heat up to 50°C, and stir for 1 hour . After the reaction was detected by TLC, the aqueous phase was removed by liquid separation, and the organic phase was washed with 10 mL of water to separate the layers. The organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain a light yellow crude product, which was washed with 20 mL ethyl acetate / petroleum ether (v / v=2:3) recrystallized to obtain 3.45g of 25-hydroxycholesterol, yield 85.6%, melting point 179.4~180.8℃, 1 HNMR (CDCl 3 ,400MHz):δ5.34(m,1H,6-CH),3.51(m,1H,3-CH),2.27(m,2...
Embodiment 2
[0031] At room temperature (20-25°C), dissolve 3.84g (10mmol) of 24-dehydrocholesterol in 20mL of chloroform, add 6.01g (100mmol) of acetic acid, 0.14g (1mmol) of aluminum trichloride, and 0.1g of concentrated hydrochloric acid, Raise the temperature to 60°C, stir and react for 8 hours, after the completion of the reaction by TLC, add 10 mL of water layer to remove the aqueous phase, add aqueous sodium hydroxide solution to the organic phase, keep the system pH ≥ 12, raise the temperature to 50°C, and stir for 1 hour. After the reaction was detected by TLC, the aqueous phase was removed by liquid separation, and the organic phase was washed with 10 mL of water to separate the layers. The organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain a light yellow crude product, which was washed with 20 mL ethyl acetate / petroleum ether (v / v=2:3) recrystallized to obtain 3.24g of 25-hydroxycholesterol with a yield of 80.5% and a melting point of 179...
Embodiment 3
[0033]At room temperature (20-25°C), dissolve 4.88g (10mmol) of 24-dehydrocholesteryl benzoate in 30mL of xylene, add 12.20g (100mmol) of benzoic acid, 34.6mg (0.1mmol) of trifluoroform Gold sulfonate (AuOTf), heat up to 80°C, stir and react for 8 hours, after the reaction is complete by TLC, add 10mL of water layer to remove the water phase, add sodium hydroxide aqueous solution to the organic phase, keep the system pH ≥ 13, heat up to 80°C , and stirred for 1 hour. After the reaction was detected by TLC, the aqueous phase was removed by liquid separation, and the organic phase was washed with 10 mL of water to separate the layers. The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated to obtain a light yellow crude product, which was washed with 20 mL of ethyl acetate / petroleum ether (v / v=2:3) Recrystallized to obtain 2.95g of 25-hydroxycholesterol with a yield of 73.4% and a melting point of 179.1~180.9℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com