A cyanine-based organic compound and its application
A technology of organic compounds and cyanines, applied in the field of fluorescent probes, can solve the problems of damaging biological samples, high detection limits, and inability to detect cysteine, so as to improve detection accuracy and reduce interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The structural formula of cyanine-based organic compounds is:
[0021]
[0022] The compound of formula I is combined with cysteine in the water body to be measured, the simulated physiological environment, or the inside and outside of the organism to obtain the compound of the structure formula II, which leads to the change of the fluorescence intensity and wavelength of the compound of formula I, as well as the change of ultraviolet absorption, and then uses The compound of formula I can detect cysteine qualitatively and quantitatively.
[0023]
[0024] Preparation of organic compound of formula I based on cyanine:
[0025] (1) Preparation of Compound 1
[0026] Dissolve 20-50mL of DMF in a graduated cylinder in 20-40mL of dichloromethane, pour it into a 250mL three-neck flask (stir with a magnet), and cool in an ice-water bath. Dissolve 20-40mL of phosphorus oxychloride into 20-40mL of dichloromethane, pour it into a constant pressure funnel, add dropwis...
Embodiment 2
[0043] The prepared formula one compound is used as a probe in the water system, simulated physiological environment and intracellular detection of cysteine, simulated physiological conditions, the following experiments are carried out under pH7-8 conditions (HEPES buffer solution, The concentration is 1-30mM), and the probe concentration is 0.5-10μM.
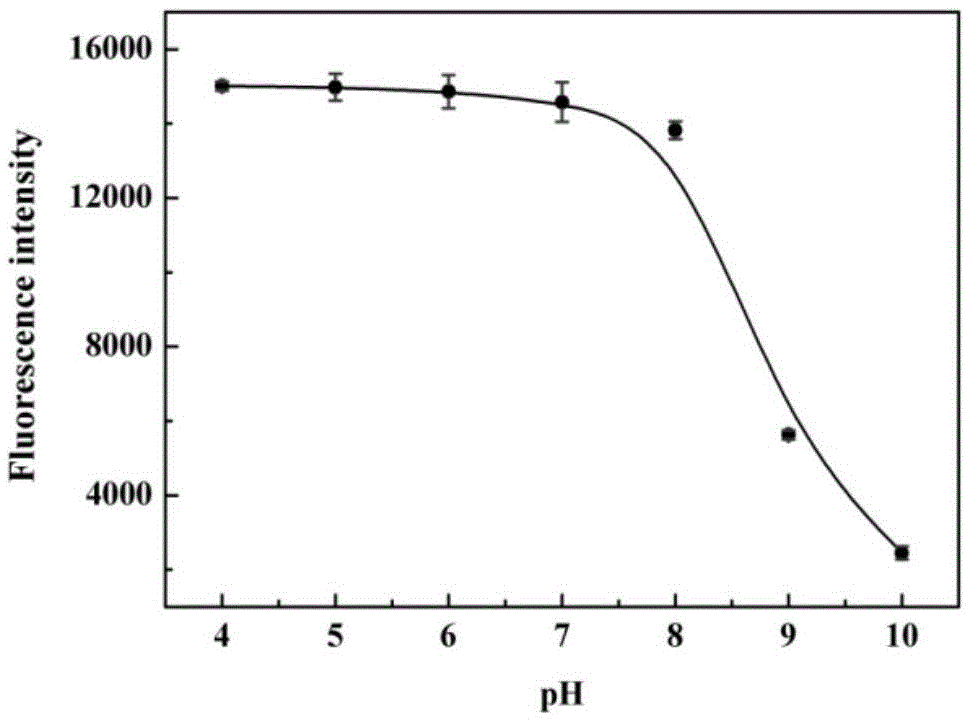
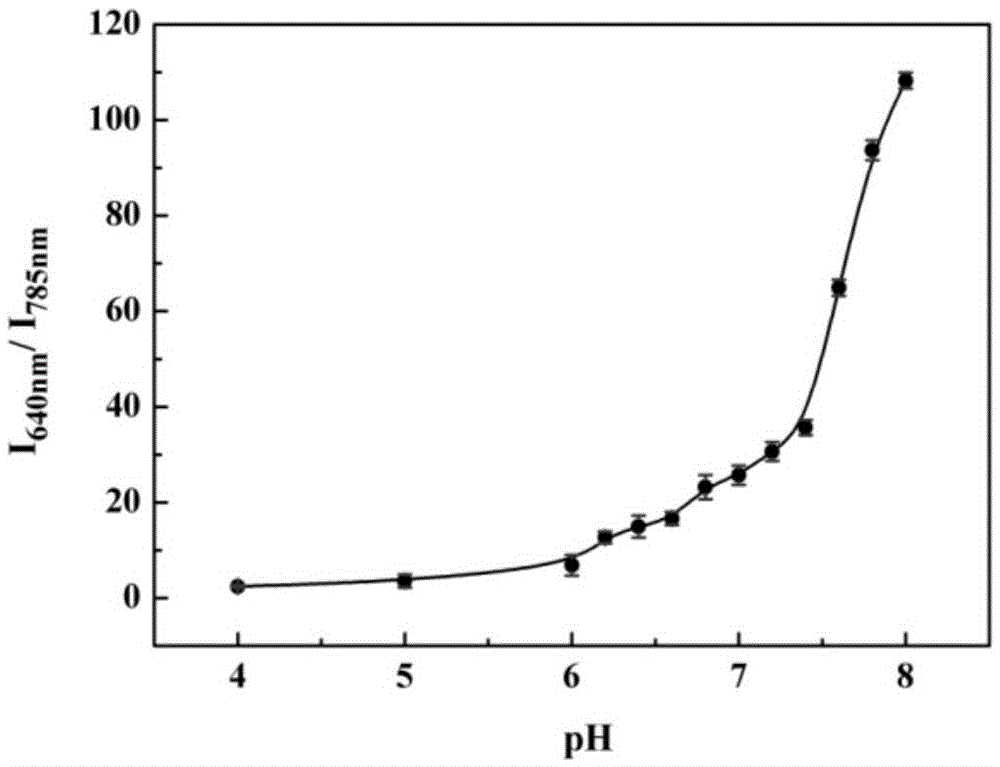
[0044] The pH effect of the probes prepared above on response to cysteine:
[0045] The pH gradient was controlled with HEPES buffer solution. Add 0.8-1mL of 1-10mM HEPES with different pH gradients into a 1.5ml colorimetric tube, then add 1-10μL of 100μM probe, shake the solution, and equilibrate at 25-40℃ for 10-30min, then add the above mixed working solution Fluorescence spectra were measured in fluorescent dishes. Fluorescence intensity changes with pH as figure 1 shown. Depend on figure 1 It is shown that the fluorescence intensity does not change significantly in the range of pH 4.0-8.0, that is, in the system of pH...
Embodiment 3
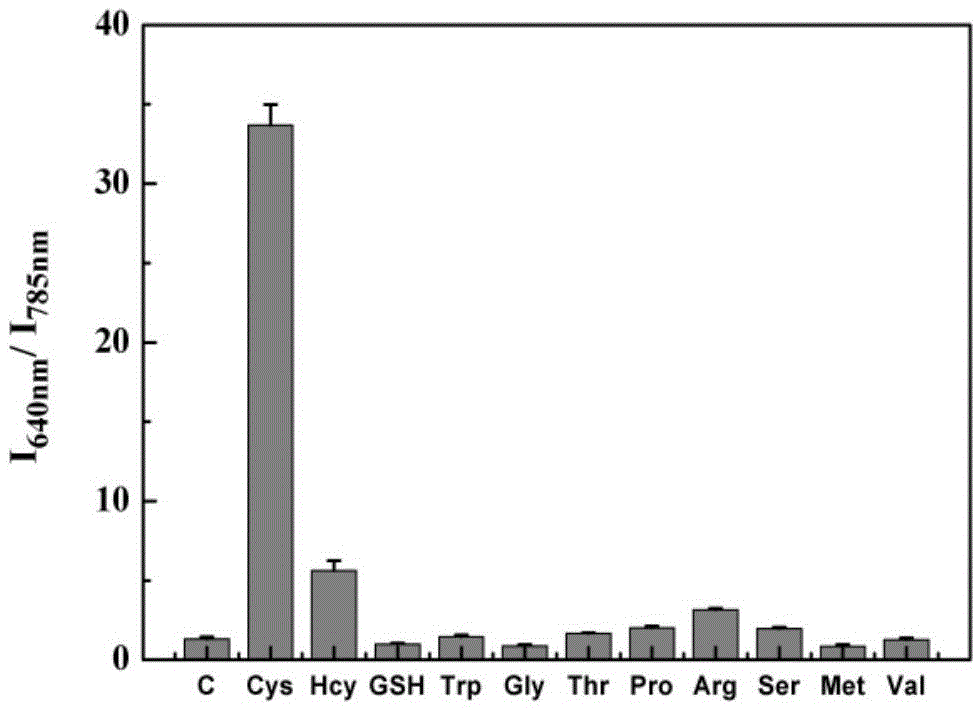
[0047] Probe selectivity for cysteine
[0048] pH was controlled with HEPES buffer solution. Take multiple 1.5ml colorimetric tubes, add 0.9-1mL 1-10mM HEPES, pH7-8 to the colorimetric tubes, then add 1-10μL 100μM probe, and then add the analytes such as figure 2 As shown, the working solution of the analyte is as follows: blank (no amino acid), cysteine, homocysteine, glutathione, tryptophan, glycine, threonine, proline, arginine , serine, methionine, valine, the final concentration of cysteine and homocysteine is 20 μM, and the final concentration of others is 2 mM. Shake the solution evenly, and after equilibrating at 25-40°C for 10-30 minutes, pour the working solution in each colorimetric tube into a fluorescent dish to measure the fluorescence spectrum. The selectivity of the probe for cysteine as figure 2 shown. It can be seen from the figure that the probe has good selectivity to cysteine, and after interacting with cysteine, the fluorescence excited at 720...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com