Organic compound based on cyanine and application thereof
A technology for organic compounds and organisms, applied in the field of near-infrared fluorescent probes, can solve the problems of slow Sec response, damage to biological samples, and large photobleaching effect of organisms, so as to reduce interference and improve detection accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of organic compound of formula I based on cyanine:
[0030] Under nitrogen protection, a commercial cyanine dye (60 mg, 0.1 mmol) was dissolved in a mixture of triethylamine (0.1 mL) and anhydrous dichloromethane (5 mL), and 2,4-dinitro Sulfonyl chloride (0.3 mmol), and the resulting mixture was stirred at 50°C for 6 hours. Then, with 10 mL saturated NaHCO 3 Aqueous solution quenched the reaction. Ethyl acetate (50 mL) was added and neutralized with hydrochloric acid (150 mL, 1M), then washed with brine. Stand still after washing, collect the organic layer and dry it with anhydrous magnesium sulfate, and concentrate in vacuo to obtain the green viscous solid of formula I, and then pass through the gradient eluent CH 2 Cl 2 and CH 3 OH (100:0-85:15, v / v) by chromatography on silica gel (200-300 mesh).
[0031] 1 H NMR (400MHz, CD 3 OD)δ(ppm):8.57(s,1H),7.87-7.79(m,16H),7.75-7.70(m,9H),7.50-7.37(m,4H),7.17-7.12(m,1H), 7.09-7.01(m,3H),6.79-6.75(d,1H),5...
Embodiment 2
[0034] The compound shown in the prepared formula I was used as a probe to detect Sec in water system, simulated physiological environment and cells, and simulated physiological conditions. The following experiments were all carried out under the condition of pH=7.4 (HEPES buffer solution, concentration 40 mM), the probe concentration was 15 μM.
[0035] The ultraviolet response of the compound shown in formula I prepared above to Sec:
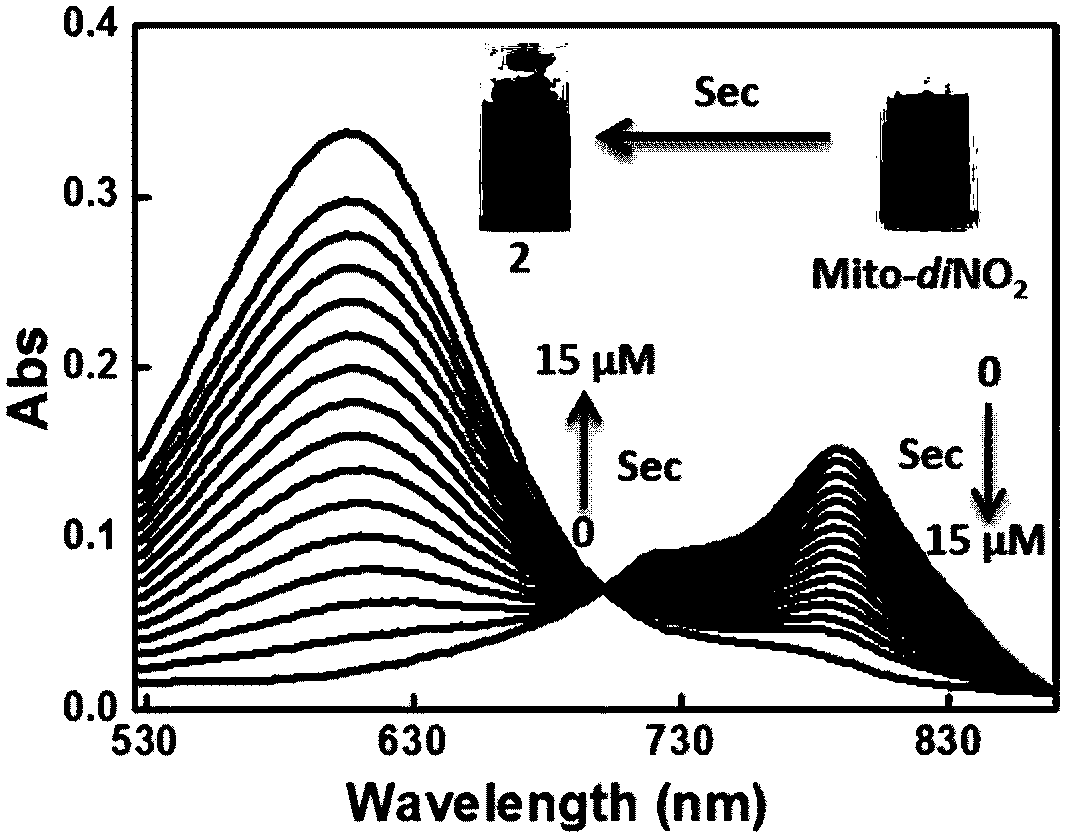
[0036] pH was controlled with HEPES buffer solution. Add 15μM compound of formula Ⅰ to each 10mL colorimetric tube, then add 40mM HEPES with different pH, then add 15μM Sec, dilute to 10ml with ultrapure water, shake the solution well, and after equilibrating for 10min, add the above working solution into the colorimetric tube The UV absorption spectrum was measured in a dish. The changes of the ultraviolet absorption spectrum before and after the detection of Sec are as follows: figure 1 As shown, the compound represented by formula I can b...
Embodiment 3
[0039] The fluorescence response of the compound shown in formula I to Sec:
[0040] pH was controlled with HEPES buffer solution. Add 15μM compound formula 1 to each 10ml colorimetric tube, then add 40mM HEPES with different pH, then add 15μM Sec, dilute to 10ml with ultra-pure water, shake the solution, after 10min equilibration, add the above working solution into the fluorescent dish Measure the fluorescence spectrum. The changes of fluorescence spectrum before and after detecting Sec are as follows: figure 2 shown. The compound represented by formula I can be used to realize Sec detection in vivo.
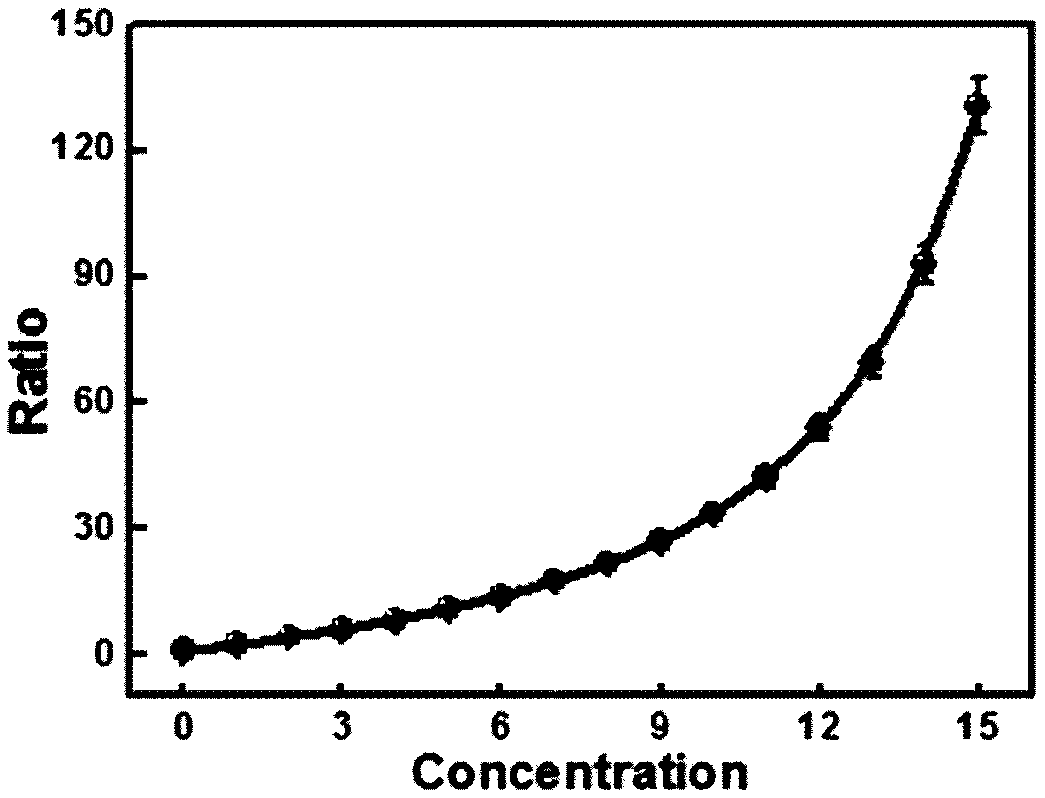
[0041] Depend on figure 2 Indicates the change of fluorescence intensity of the system with the change of Sec concentration, indicating that with the increase of Sec concentration, the fluorescence intensity of the system in the 630-830nm band is obviously enhanced, and the fluorescence intensity of the 780-810nm band is obviously weakened.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com