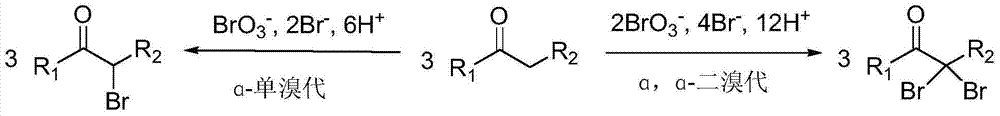
Method for preparing alpha-monobrominated ketone and alpha, alpha-dibrominated ketone compounds by selectively brominating ketone compounds
A ketone compound, monobromination technology, applied in the field of organic compound preparation, can solve the problems of easy volatility, low reactivity, unsafe operation, etc., and achieves high reaction rate and yield, easy industrial production, and bromination selectivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of α-bromoacetophenone from selective monobromination of acetophenone:
[0029] Acetophenone 10mmol, Potassium Bromate 3.3mmol, Hydrobromic acid 10mmol, Methanol 10mL, 60°C, Stir for 2.5h to stop the reaction, Spin off and recover Methanol, add 10mL of ethyl acetate to dissolve, wash with 30mL of water three times, dry over anhydrous sodium sulfate . Filtration and rotary evaporation gave white solid α-bromoacetophenone with a yield of 95%. 1 H NMR (CDCl 3 ,300MHz)δ8.00-8.03(m,2H,ArH),7.68-7.70(m,1H,ArH),4.44(s,2H,CH 2 Br).
Embodiment 2
[0031] Preparation of α-bromo-4-chloroacetophenone from selective monobromination of 4-chloroacetophenone:
[0032] 10mmol of 4-chloroacetophenone, 3mmol of sodium bromate, 6mmol of sodium bromide, 11mmol of sulfuric acid, 10mL of ethanol, 70°C, stirred for 1.5h, and then stopped the reaction. Three times, dried over anhydrous sodium sulfate. Filtration and rotary evaporation gave α-bromo-4-chloroacetophenone with a yield of 94%. 1 H NMR (CDCl 3 ,300MHz)δ7.94(d,J=8.4Hz,2H,ArH),7.48(d,J=8.4Hz,2H,ArH),4.41(s,2H,CH 2 Br).
Embodiment 3
[0034] Preparation of α-bromo-3-bromoacetophenone from selective monobromination of 3-bromoacetophenone:
[0035]10mmol of 3-bromoacetophenone, 3.5mmol of sodium bromate, 7mmol of sodium bromide, 8mmol of phosphoric acid, 10mL of ethanol, at 70°C, stir for 2 hours and then stop the reaction. Three times, dried over anhydrous sodium sulfate. Filtration and rotary evaporation gave α-bromo-3-bromoacetophenone with a yield of 91%. 1 H NMR (CDCl 3 ,300MHz)δ7.20–8.20(m,4H,ArH),4.38(s,2H,CH 2 Br).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com