Method for preparing polymer containing cucurbituril structure
A polymer, cucurbituril technology, applied in the field of polymer preparation, can solve the problems of poor solubility of cucurbituril, difficulty in functionalization of cucurbituril, etc., and achieve the effect of good biocompatibility, excellent performance, and good recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
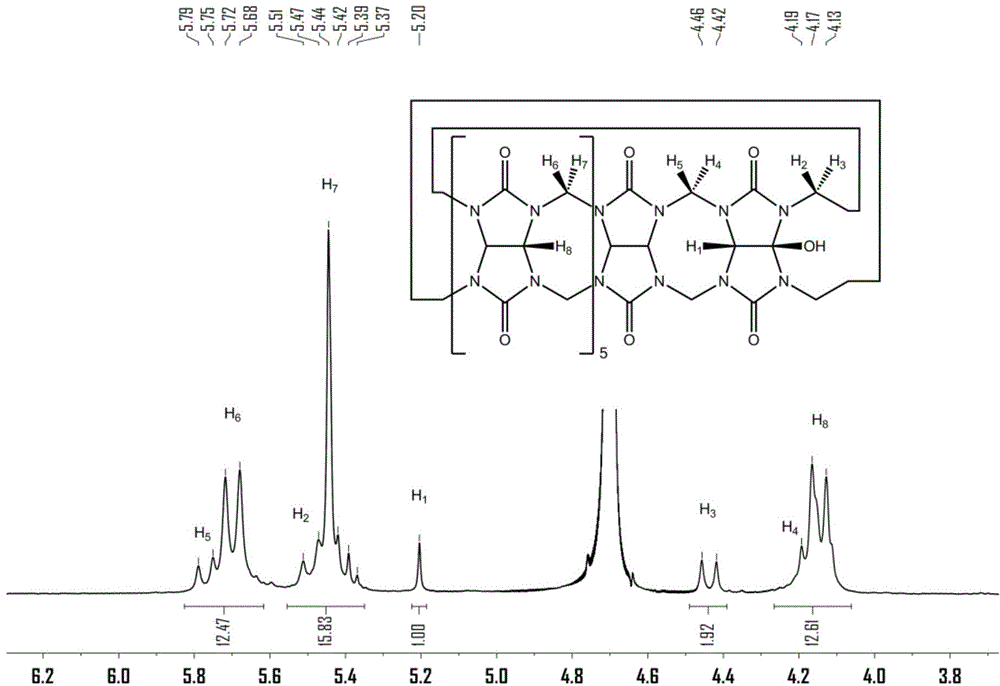
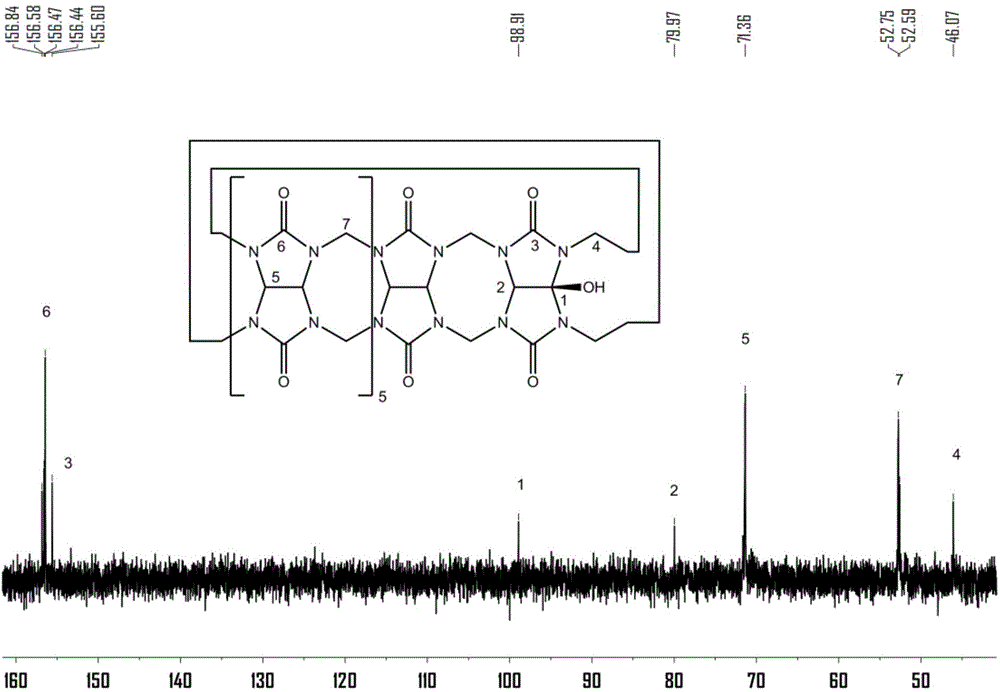
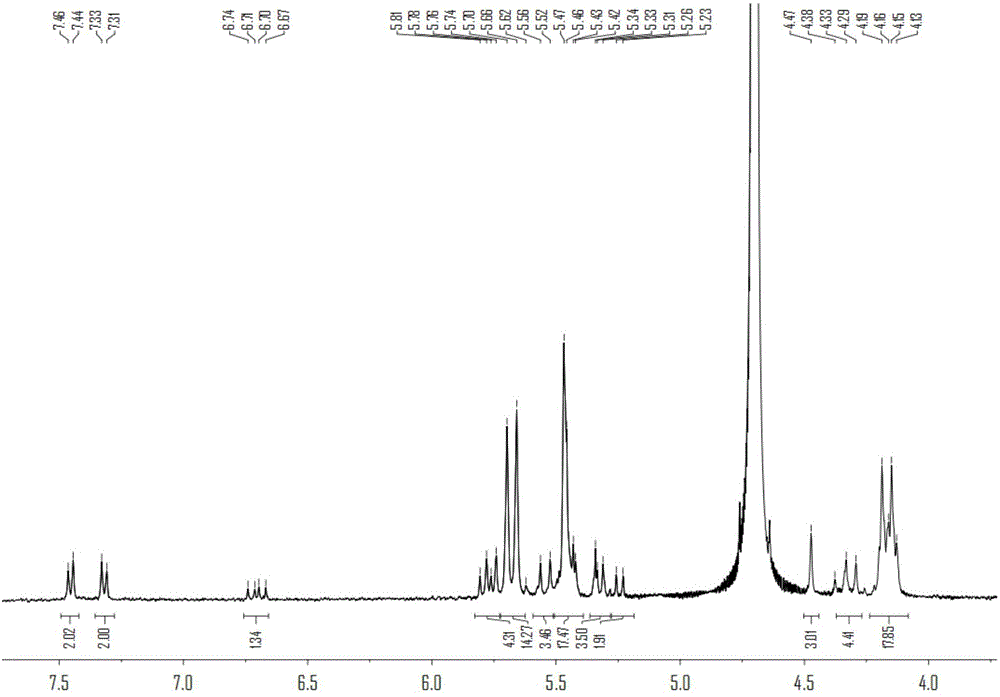
[0056] Preparation of polymers containing cucurbit [7] urea structure:
[0057] (1) Cucurbit[7]urea 0.5g was dispersed in 50mL ultrapure water, and 0.2g of 3,3'-(1,8-dioctamethylene)-bis-(1-ethylimidazole)dibromide was added at 50 Heat and stir at ℃ for 30 minutes to form a homogeneous solution;
[0058] (2) After the homogeneous solution obtained in step (1) was bubbled with nitrogen for 15 minutes, 0.12 g of ammonium persulfate was added, and heated to 85°C for oxidation reaction; Resin chromatography column separation, obtains compound 0.142g shown in formula (II);
[0059] (3) 0.1 g of the compound shown in formula (II) was dissolved in 30 mL dimethyl sulfoxide, 0.05 g of sodium hydride was added at 0° C., and reacted for 5 h; then 0.2 g of 4-vinyl chloride was added, and reacted for 16 h; after the reaction, Add ether to precipitate the product, and wash it with methanol to obtain the complex of 4-vinylbenyloxycucurbituril and 3,3'-(1,8-dioctamethylene)-bis-(1-ethylimid...
Embodiment 2
[0071] As described in Example 1, the difference is that the 4-vinylbenyloxycucurbit[7]urea in step (5) is 0.2 g, the water is 30 mL, and the 3,3'-(1,8-dioctyl )-bis-(1-ethylimidazole) dibromide 0.10g; in step (6), the monomer was changed to 1.2g acrylamide, and the initiator was 0.005g ammonium persulfate and 0.0024g sodium bisulfite.
Embodiment 3
[0073] As described in Example 1, the difference is that the 4-vinylbenyloxycucurbit[7]urea in step (5) is 0.1 g, the water is 20 mL, and the 3,3'-(1,8-dioctyl )-bis-(1-ethylimidazole) dibromide 0.04g; in step (6), the monomer was changed to 0.5g acrylamide, and the initiator was 0.003g ammonium persulfate and 0.0015g sodium bisulfite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com