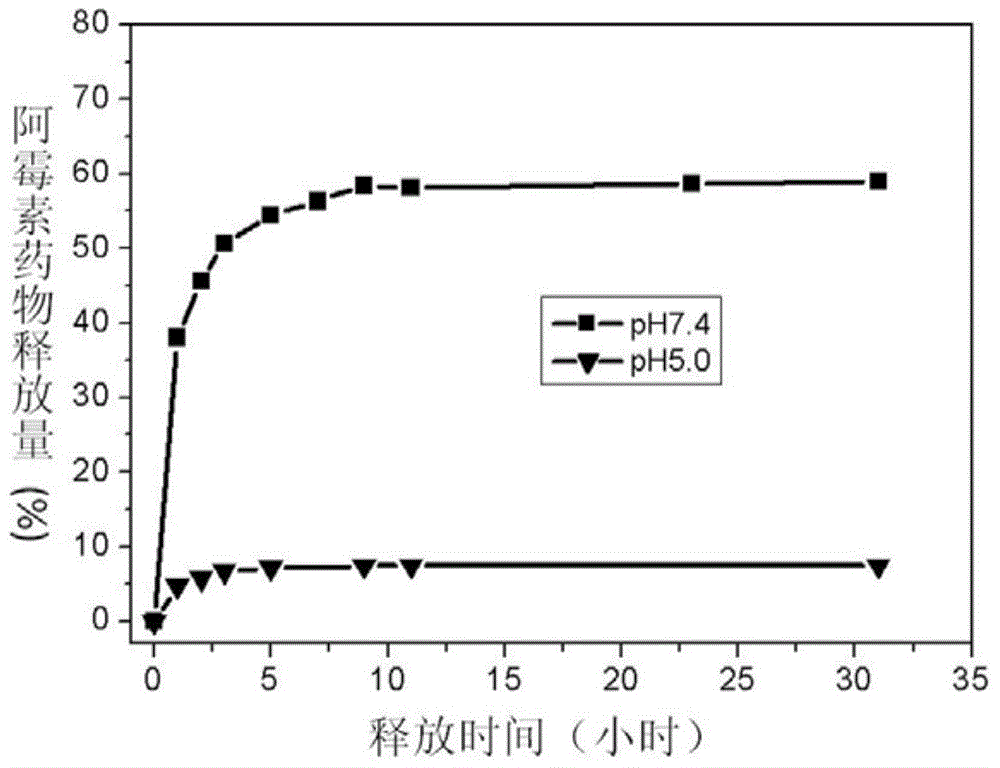
Nano-medicinal carrier with magnetocaloric effect as well as preparation method and application thereof
A nano drug carrier and magnetocaloric effect technology, applied in the field of medicine, can solve the problems of reducing drug efficacy, poor selectivity of chemotherapy drugs, killing normal cells, etc., achieve good delivery efficiency, increase storage capacity, and reduce complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The nano-drug carrier provided in this example includes mesoporous silica particles and Fe embedded in the mesoporous silica particles. 3 o 4nanoparticles. The nano drug carrier preparation method comprises the following steps:
[0036] Step 1: Take FeCl 3 ·6H 2 O and FeCl 2 4H 2 O each 19.2 mmoles, after being dissolved in 25 milliliters of deionized water, add 0.85 milliliters of concentrated hydrochloric acid (36-38%); Then 250 milliliters of 1.5 mol / liter sodium hydroxide solution is added dropwise in the above solution, and Stirring at room temperature at a stirring speed of 700 rpm for 1 hour produced a black precipitate.
[0037] Step 2: Use a magnet block to collect the black precipitate, wash the black precipitate three times with deionized water, then wash three times with ethanol, and finally dry it in vacuum at 60°C for 24 hours to obtain Fe 3 o 4 nanoparticles.
[0038] Step 3: Take 0.4 grams of Fe 3 o 4 Nanoparticles were ultrasonically disperse...
Embodiment 2
[0045] The nano-drug carrier provided in this example is an amino-modified nano-drug carrier, and the silane coupling agent used is γ-aminopropyltriethoxysilane, and its preparation method is as follows:
[0046] Take 1 gram of the nano drug carrier prepared in Example 1, ultrasonically disperse it in absolute ethanol to obtain a suspension, then quickly add 3 ml of γ-aminopropyltriethoxysilane to the above suspension, seal the container and stir at room temperature for 24 hours, and finally wash off the unreacted γ-aminopropyltriethoxysilane with absolute ethanol and vacuum-dry to obtain the amino-modified nano-drug carrier.
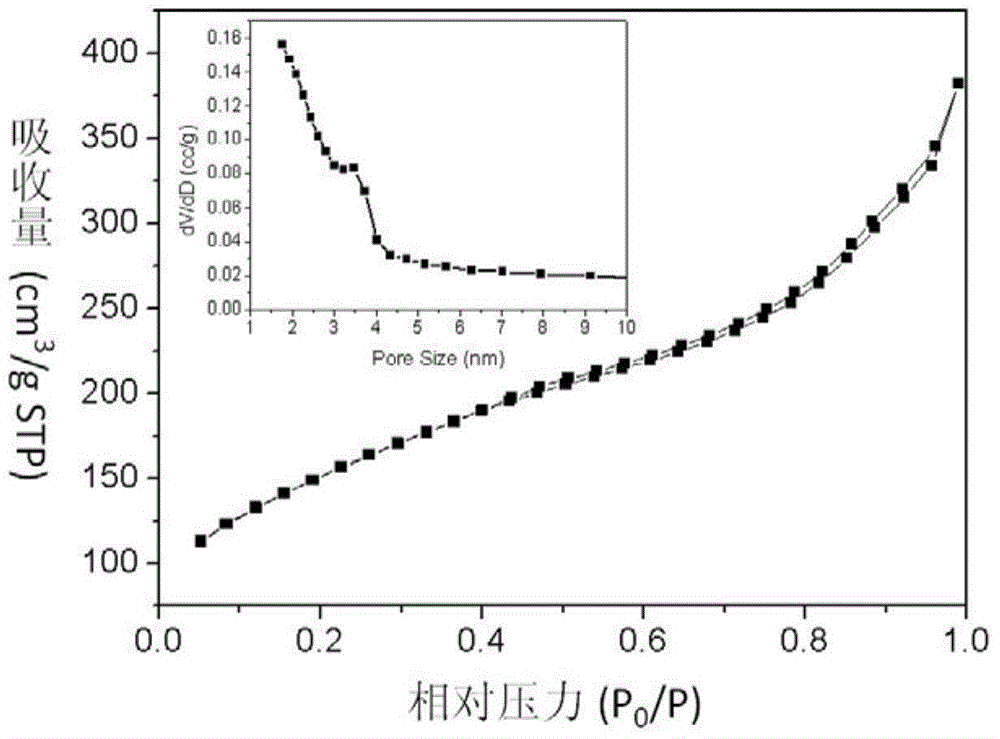
[0047] The particle diameter of the amino-modified nano drug carrier is about 150 nanometers, and the average mesoporous diameter of the mesoporous silicon oxide particles is 3.2 nanometers.
[0048] In addition, the silane coupling agent used in this example is γ-aminopropyltrimethoxysilane, and the outer surface of the mesoporous silica particles invo...
Embodiment 3
[0050] The nano-drug carrier provided in this example is a nano-drug carrier modified by RBITC, which is used to observe the uptake of the nano-drug carrier by cells under a fluorescence microscope. The silane coupling agent that adopts is RBITC-APTES silane coupling agent, and its preparation method is:
[0051] Take 15 mg of Rhodamine B isothiocyanate (Rhodamine B isothiocyanate, RBITC) and 100 microliters of γ-aminopropyltriethoxysilane, add 5 milliliters of absolute ethanol, and stir in a sealed container under dark room conditions for 24 Hours, the rhodamine B modified silane coupling agent (RBITC-APTES) was obtained.
[0052] Then, take 20 mg of the nano-drug carrier prepared in Example 1, disperse it in 6 ml of absolute ethanol, add 1 ml of RBITC-APTES silane coupling agent, and stir in a sealed container for 24 hours in a dark room. Finally, the above particle solution was centrifuged, washed with absolute ethanol for several times to remove the unreacted RBITC-APTES ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mesopore diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com