Method for taking 3-aryl-5-methyl butyrolactone compound as acetylglucosyl bromide
An anti-plant virus agent, methyl butyrolactone technology, applied in botany equipment and methods, plant growth regulators, disinfectants, etc., can solve the problem that the inhibitory performance has not been seen in relevant reports, and achieve high bactericidal activity, Good bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
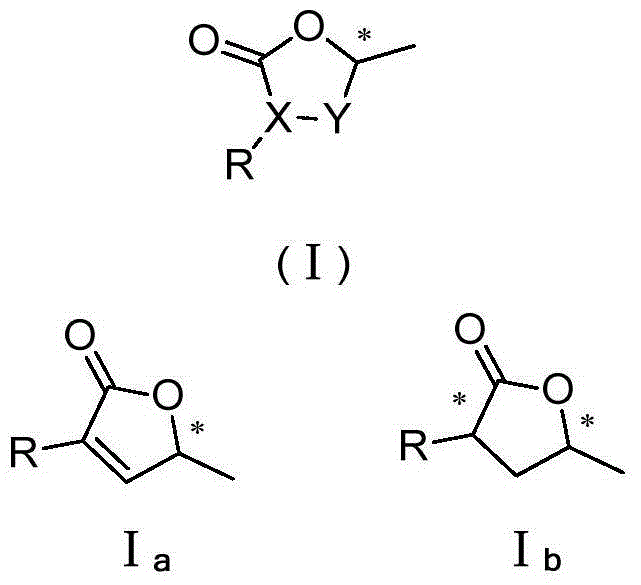
[0036] A. The described 3-aryl-5-methylbutyrolactone compound is a compound having the structure shown in the general formula (I) as shown below:
[0037]
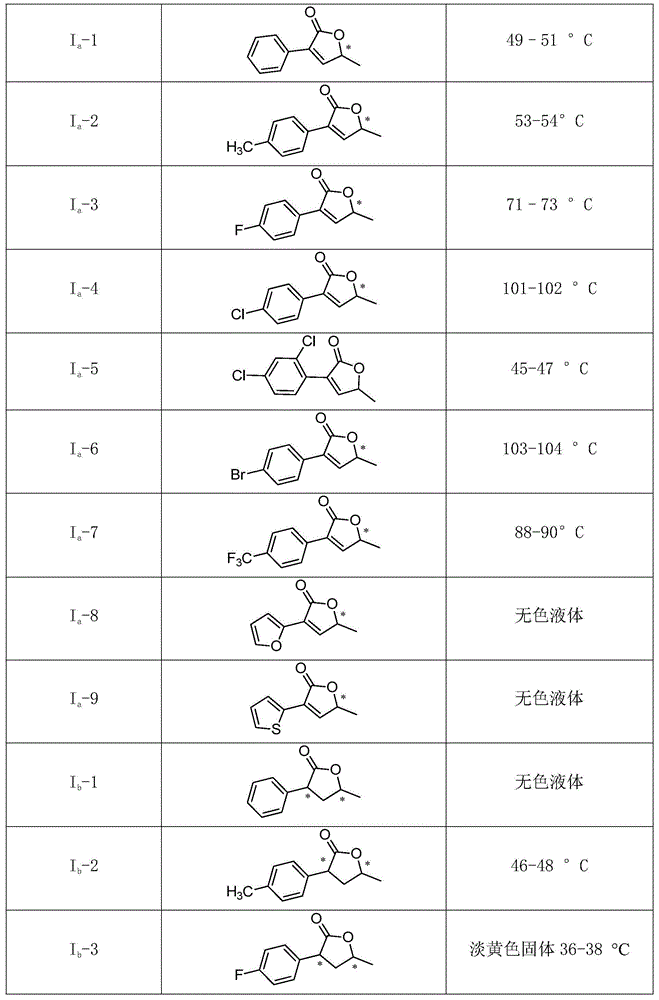
[0038] The compound of structure shown in general formula (I) comprises I again a and I b Two categories, the specific individual compounds are as follows:
[0039]
[0040]
[0041] B. The method that above-mentioned 3-aryl-5-methylbutyrolactone compound is used as anti-plant virus agent is as follows:
[0042] B-1. The steps for anti-tobacco mosaic virus activity are as follows:
[0043] The first step, tobacco mosaic virus purification and concentration determination:
[0044] Tobacco mosaic virus purification and concentration determination were carried out in accordance with the Tobacco Mosaic Virus SOP specification compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. The virus crude extract was centrifuged twice with polyethylene glycol, then the concentration was measured...
Embodiment 2
[0064] Except the individual compound I of the above-mentioned 3-aryl-5-methylbutyrolactone compound in the second step a In the preparation of -1 medicament solution, weigh the individual compound I of the above-mentioned 3-aryl-5-methylbutyrolactone compound a -140mg is used as the original drug, and then DMF0.4mL is added to the original drug to dissolve, and 1×10 5 μg / mL mother liquor, then dilute with the Tween 80 aqueous solution that mass percent concentration is 1 ‰ to the test concentration except that 100 μg / mL, other are the same as embodiment 1.
Embodiment 3
[0066] Individual compounds other than the above-mentioned 3-aryl-5-methylbutyrolactone compounds are I a -2, other with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com