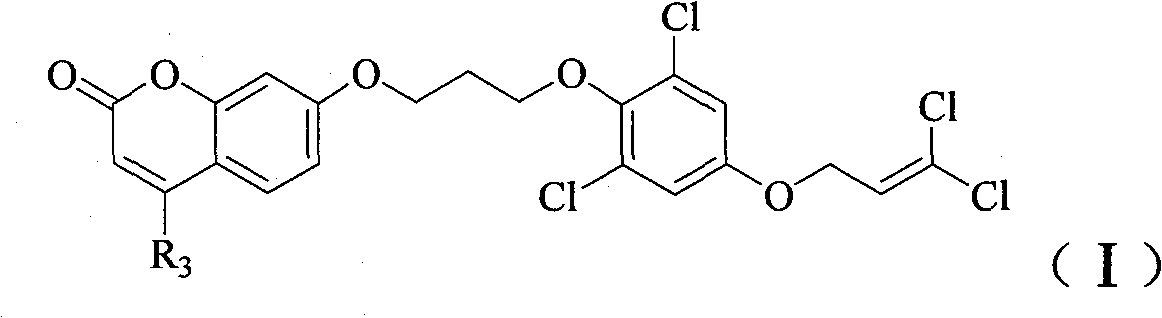
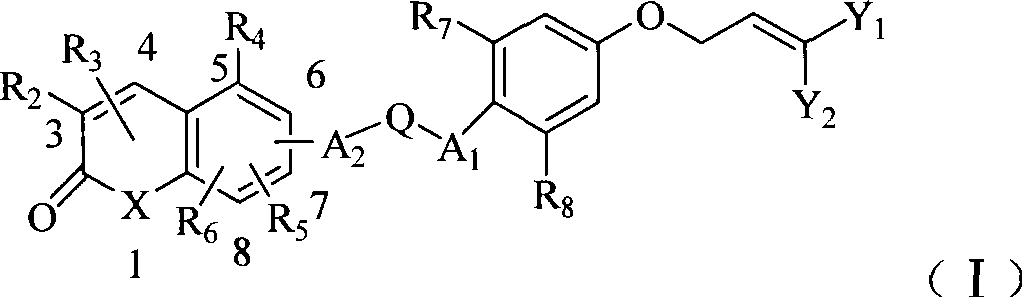
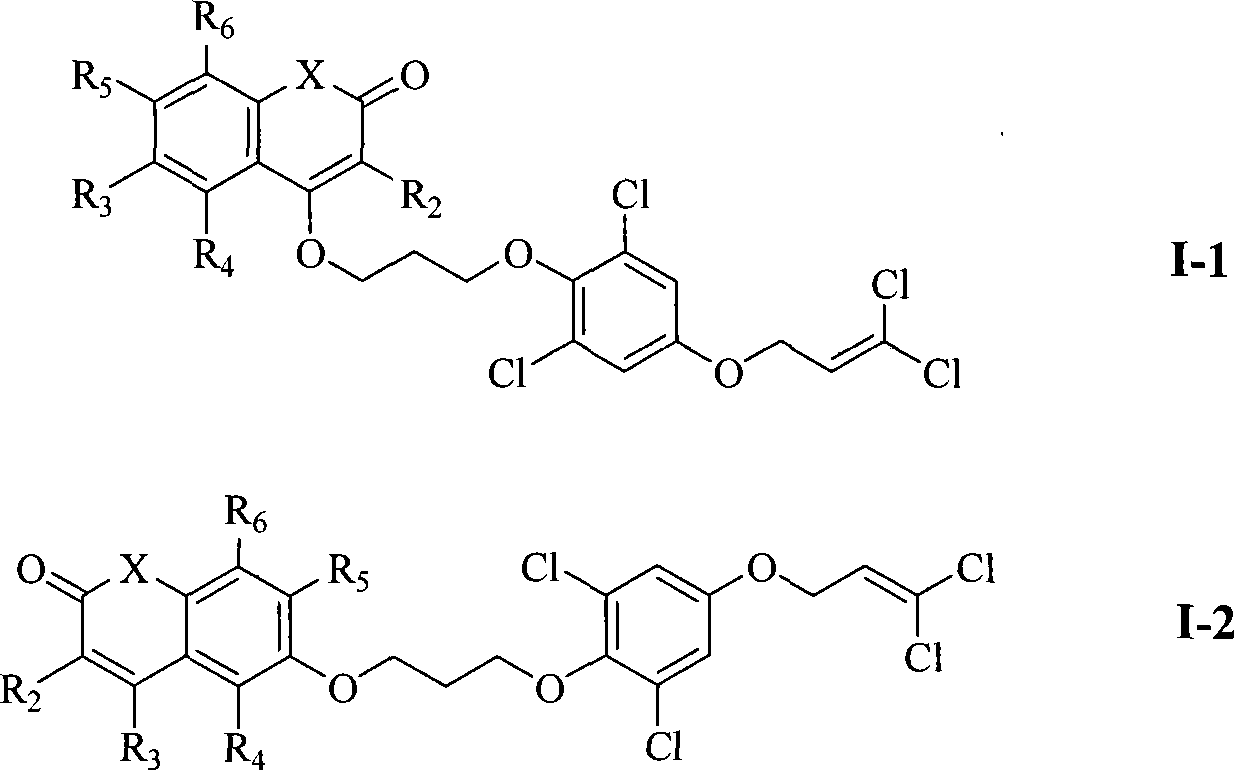
Substituent ether compound and application thereof
A compound, substituted ether technology, applied in the field of substituted ether compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0184] Example 1: Preparation of Compound 2
[0185]
[0186] Take 0.18 g of III-1 and 0.23 g of anhydrous potassium carbonate in a 100 ml reaction flask, add 40 ml of N,N-dimethylformamide (DMF), add 0.45 g of IV-1 under stirring, heat up to 35°C and stir React for 3 hours. After the completion of the TLC monitoring reaction, after desolvation under reduced pressure, pour 50 milliliters of saturated saline into the reaction flask, extract three times with 60 milliliters of ethyl acetate, dry, desolventize, and obtain 0.33 grams of product by column chromatography, namely compound 2.
example 2
[0187] Example 2: Preparation of Compound 76
[0188]
[0189] Take 0.23 g of III-2 and 0.23 g of anhydrous potassium carbonate in a 100 ml reaction flask, add 40 ml of N,N-dimethylformamide (DMF), add 0.45 g of IV-1 under stirring, heat up to 35°C and stir React for 3 hours. After the completion of the TLC monitoring reaction, after desolvation under reduced pressure, pour 50 milliliters of saturated saline into the reaction flask, extract three times with 60 milliliters of ethyl acetate, dry, desolventize, and obtain 0.33 grams of product by column chromatography, namely compound 76.
[0190] Other compounds of general formula (I) can be prepared by the preparation method provided by the present invention.
[0191] Melting points and NMR data of some compounds ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0192] Compound 2: melting point 78-80°C. δppm 7.51(d, 1H), 6.85(m, 4H), 6.13(m, 2H), 4.58(d, 2H), 4.35(t, 2H), 4.16(t, 2H), 2.41(s, 1H), ...
preparation Embodiment
[0195] Formulation example (the addition amount of each component is the weight percent content, and the active compound is metered in after 100 percent)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com