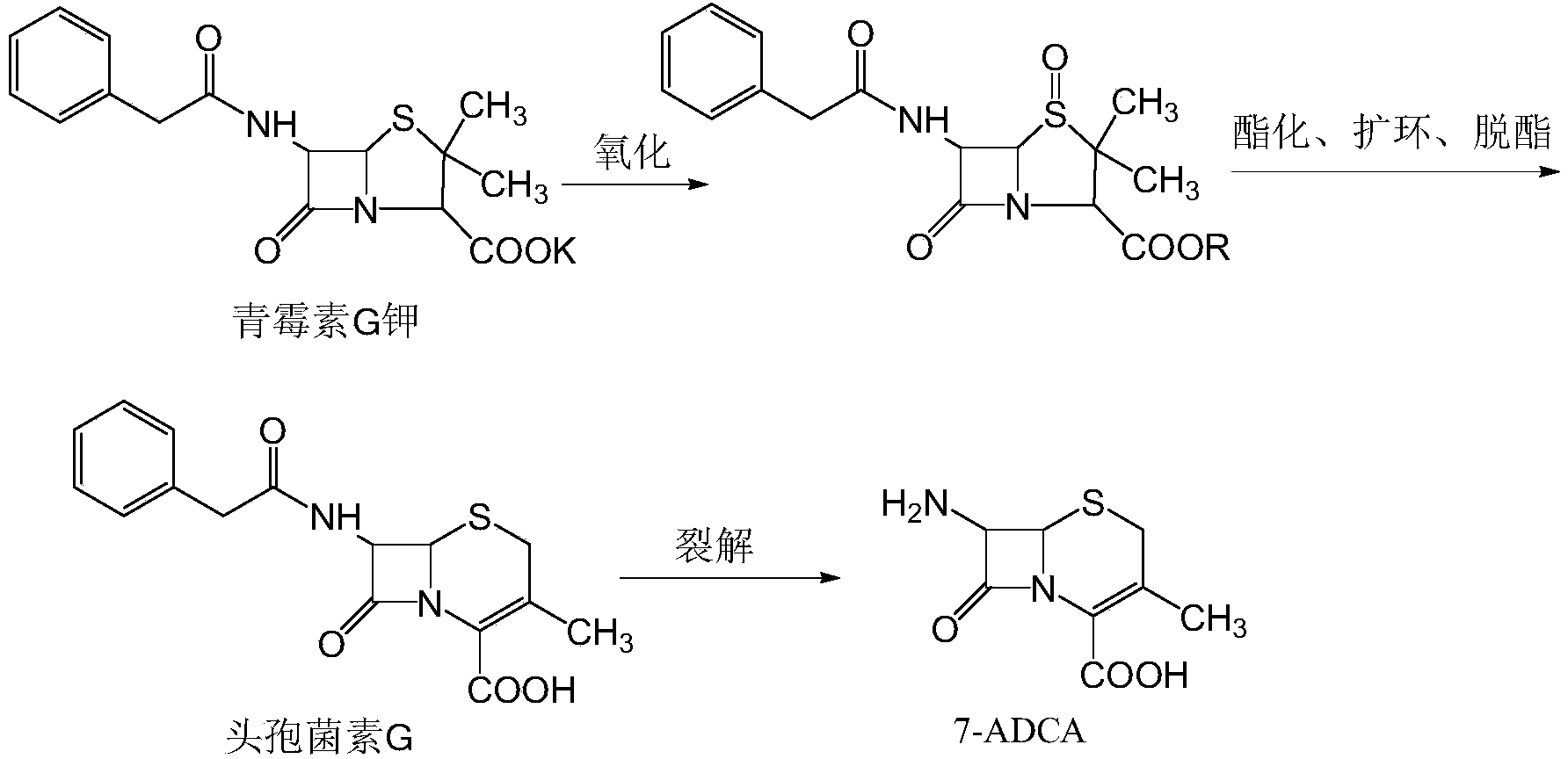
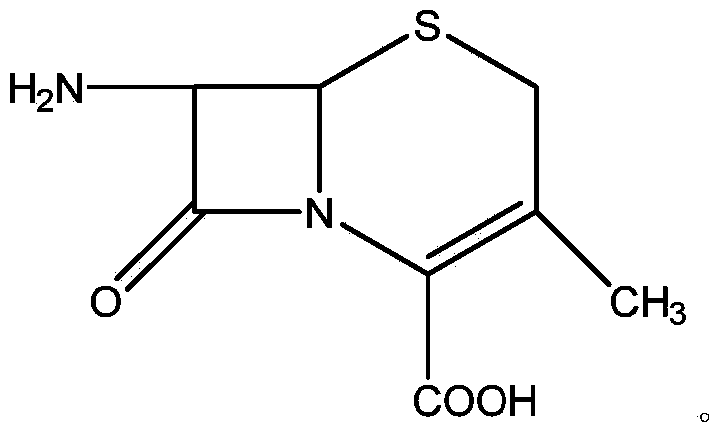
Preparation method of 7-ADCA
A 7-ADCA and temperature control technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield, many steps, and high requirements for reaction conditions, and achieve the effect of high yield, few operation steps, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Oxidation
[0049] Dissolve 37.2g of penicillin G potassium in water, adjust the pH to about 3 with dilute sulfuric acid, slowly add 10.2g of 40% hydrogen peroxide dropwise, control the temperature at about 1-3°C, and control the reaction end point with thin-layer chromatography or HPLC. Add 2.0mol / L sulfuric acid after the reaction, when the reaction solution becomes white and turbid, add dilute sulfuric acid to pH 1.5, let it stand, filter, wash the filter cake with ice water, and dry it in vacuum to obtain 34.6g white powdery solid , yield 93%.
[0050] 2. Preparation of cephalosporin G
[0051] (1) Preparation of BSU
[0052] Using 500ml of toluene as solvent, add 31.5g of urea, 0.4g of saccharin, and distill at normal pressure. When the water content is controlled to 0.025%, add 116ml of hexamethyldisilazane, heat up and reflux, control the temperature at 110°C, and react for 4 hours , concentrated under normal pressure, and about 150ml of toluene was distill...
Embodiment 2
[0058] 1. Oxidation
[0059] Dissolve 55.4g of penicillin G potassium in water, adjust the pH to about 3 with dilute sulfuric acid, slowly add 13.85g of 40% hydrogen peroxide dropwise, control the temperature at 1-3°C, and control the reaction end point with thin-layer chromatography or HPLC. After the reaction, add 2.0mol / L sulfuric acid, when the reaction solution becomes white and turbid, add dilute sulfuric acid until the pH is 2, let it stand, filter, wash the filter cake with ice water, and dry it in vacuum to obtain 52.2g of white powdery solid , yield 94.2%.
[0060] 2. Preparation of cephalosporin G
[0061] (1) Preparation of BSU
[0062] Using 500ml of toluene as solvent, add 31.5g of urea, 0.315g of saccharin, and distill at normal pressure. When the water content is controlled to 0.02%, add 116ml of hexamethyldisilazane, heat up and reflux, control the temperature at 110°C, and react for 4 hours , concentrated under normal pressure, distilled off about 150ml of...
Embodiment 3
[0068] 1. Oxidation
[0069] Dissolve 37.2g of penicillin G potassium in water, adjust the pH to about 3 with dilute sulfuric acid, slowly add 11.16g of 40% hydrogen peroxide dropwise, control the temperature at 1-3°C, and control the reaction end point with thin-layer chromatography or HPLC. After the reaction, add 2.0mol / L sulfuric acid, when the reaction solution becomes white and turbid, add dilute sulfuric acid to pH 1.7, let it stand, filter, wash the filter cake with ice water, and dry it in vacuum to obtain 34.4g white powdery solid , yield 92.5%.
[0070] 2. Preparation of cephalosporin G
[0071] (1) Preparation of BSU
[0072] Using 500ml of toluene as solvent, add 31.5g of urea, 0.473g of saccharin, and distill at normal pressure. When the water content is controlled to 0.015%, add 116ml of hexamethyldisilazane, heat up and reflux, control the temperature at 110°C, and react for 4 hours , concentrated under normal pressure, and about 150ml of toluene was distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com