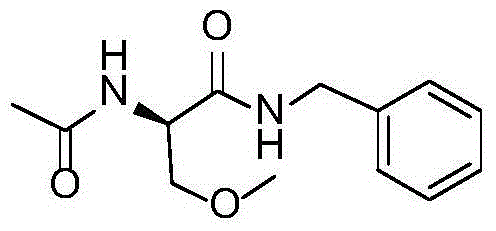
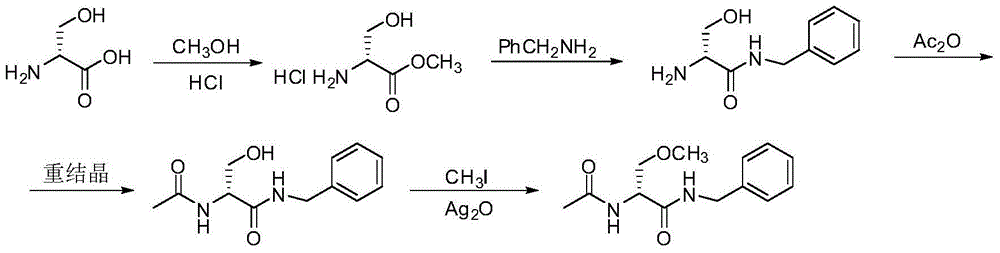
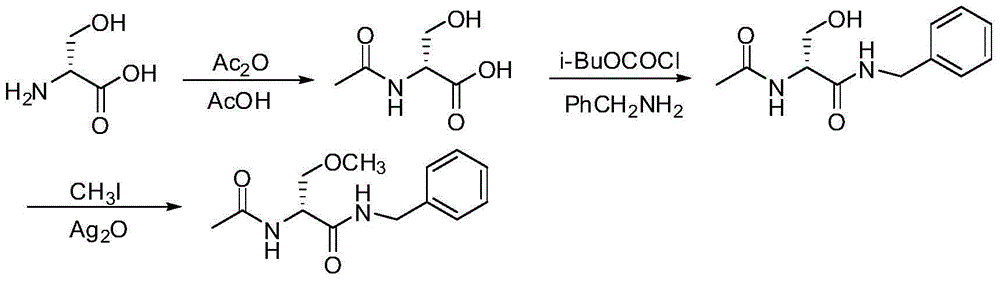
A preparing process of lacosamide
A technology for lacosamide and compounds, which is applied in the field of preparation of lacosamide, can solve the problems of many by-products, troublesome post-processing, unfavorable industrial production, etc., and achieve the effect of high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1: Preparation of N-Boc-D-serine (compound of formula I)
[0096] Dissolve sodium hydroxide (8.4g, 0.210mol) in water (53ml) at room temperature, cool to 5-10°C, and add D-serine (10.5g, 0.100mol) and di-tert-butyl dicarbonate at ≤10°C Ester (26.2 g, 0.120 mol) was heated to 30-35 ° C for 20 hours to obtain an aqueous solution of the compound of formula I (the yield was 100%, HPLC purity 99.1%, chiral purity 99.4%). The compound of formula I does not need to be separated, and the reaction solution is directly used for the next step of synthesis.
[0097] HPLC purity testing conditions and methods:
[0098] HPLC detection conditions: Instrument: Shimadzu LC-20A high performance liquid chromatograph; Chromatographic column: C18 column, 250×4.6mm, 5μm; Mobile phase: 0.1mol / L diammonium hydrogen phosphate (phosphoric acid to adjust the pH value to 6.2) - methanol (65:35); detection wavelength: 210nm; flow rate: 1ml / min;
[0099] Determination method: Take an a...
Embodiment 2
[0103] Embodiment 2: Preparation of N-Boc-O-methyl-D-serine (compound of formula II)
[0104] The aqueous solution of the compound of formula I (20.5 g, 0.100 mol) prepared above was cooled to 0-10°C. While maintaining 0-10°C, dimethyl sulfate (50.5g, 0.400mol) and 50% sodium hydroxide (36.0g, 0.450mol) were added dropwise, and the reaction mixture was reacted at 0-10°C for 6 hours. After the reaction is completed, the reaction solution is kept at 0-10°C, acidified with 50% citric acid to pH=2-3, then extracted with dichloromethane (1×123ml, 2×82ml), dried with anhydrous sodium sulfate, and then distilled under reduced pressure To dryness, 21.9 g of the compound of formula II was obtained (yield 100%, HPLC purity 92.9%, chiral purity 98.0%).
[0105] HPLC purity testing conditions and methods:
[0106] Detection conditions: HPLC method; instrument: Shimadzu LC-20AT high performance liquid chromatography; column: CHIRALPAK AD-H (4.6mm×250mm, 5μm); column temperature: 30°C; de...
Embodiment 3
[0112] Example 3: Preparation of N-Boc-O-methyl-D-serine pivalic anhydride (compound of formula III)
[0113] The compound of formula II (21.9g, 0.100mol) obtained above was dissolved in dichloromethane (110ml), cooled to 0°C, and pivaloyl chloride (12.6g, 0.100mol) was added at 0-5°C. Add N-methylmorpholine (11.1g, 0.110mol) at 5°C and react at 0-5°C for 1 hour to obtain a methylene chloride solution of the compound of formula III. The methylene chloride solution of the compound of formula III is directly used without treatment synthesized in the next step.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com