Humanized antibody targeted to RANKL (Receptor Activator Of Nuclear Factor Kappa B Ligand) and TNF-alpha (Tumor Necrosis Factor) and application of humanized antibody
A humanized antibody and targeting technology, applied in the fields of genetic engineering and immunology, can solve limitations and other problems, achieve the effect of inhibiting differentiation and alleviating bone loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Cloning and Determination of the cDNA Sequence of the Variable Region of Animal Immunization Mouse Origin in Example 1
[0066] Extract the total mRNA (SV Total RNA, Promega) of hybridoma cells (preservation number is CGMCC NO.6853) according to the operation procedure; RT-PCR reverse transcription system (promega) obtains the cDNA of the variable region of the mouse antibody; according to the following procedures murine antibody variable region gene amplification
[0067] 1. Primers (synthesized by INVIVOGEN)
[0068] (1) Heavy chain upstream primer:
[0069] 5'-CCGCTCGAGTCATGCTCTTCTTGGTAGCAACAGCT-3'
[0070] (2) Heavy chain downstream primer:
[0071] 5'-CTAGCTAGCTGCAGAGACAGTGAGAGTGG-3'
[0072] (3) Light chain upstream primer:
[0073] 5'-CATGCCATGGAAAATGGAGACAGACACACTCCTGC-3'
[0074] (4) Light chain downstream primer: 5'-CTACGTACGCCCGTTTGATTTCCAACTT-3'
[0075] 2. PCR reaction cycle conditions
[0076] The PCR conditions for the heavy chain were: 94°C, 2min...
Embodiment 2
[0081] Example 2 Design and Synthesis of Humanized Antibody Variable Region
[0082] Design of recombinant humanized antibody: compare the heavy chain and light chain sequences of the murine antibody variable region obtained in Example 1 with the database information of the human antibody heavy chain and light chain in the BLAST program (http: / / www.ncbi.nlm.nih.gov / ). The sequences of the heavy chain (pdb:3DGG_B) and light chain (pdb:1I9R_L) of the humanized antibody with the highest homology to the variable region heavy chain and light chain of the murine antibody were respectively established.
[0083] Sequences of the heavy and light chains of the humanized antibody screened were analyzed by the VBASE2 program (http: / / www.vbase2.org / ), and the structures of their CDR and FR regions were respectively established: the CDR1 of 3DGG_B is GYTFTSYV, CDR2 is NPYNDDT, CDR3 is CAREDYYGSRWGYW. The CDR1 of 1I9R_L is QRVSSSTYSY, the CDR2 is IKAS, and the CDR3 is HSWEIPPTF. While fu...
Embodiment 3
[0084] Example 3 Construction and expression of recombinant humanized antibody expression vector
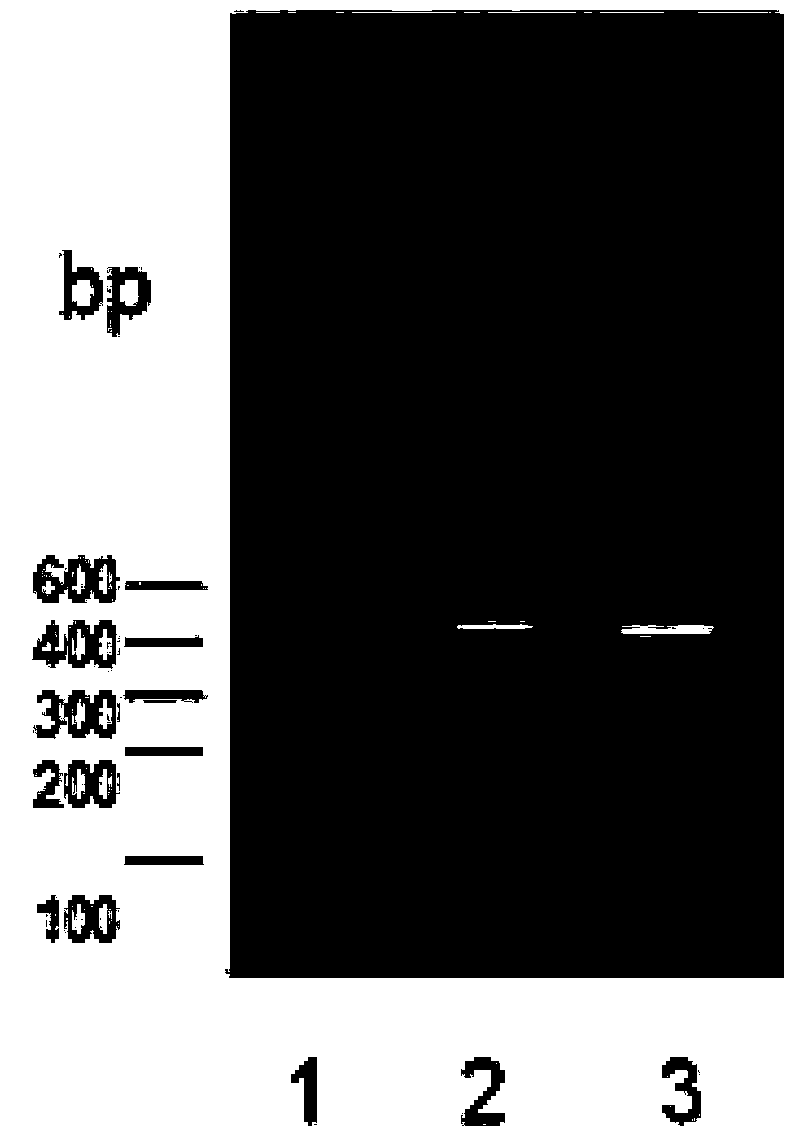
[0085] Construction of recombinant humanized antibody expression vector: The recombinant antibody heavy chain variable region sequence and pFUSEss-CHIg-hG1 (Catalog#pfusess-hchg1, purchased from InvivoGen, USA) were applied with restriction enzymes XhoI and NheI in a 37°C water bath for 1 hours for double digestion. The light chain variable region sequence of the recombinant antibody and pFUSE2ss-CLIg-hk (Catalog#pfuse2ss-hchlk, purchased from InvivoGen, USA) were subjected to double digestion with restriction enzyme NcoI in a 37°C water bath for 1 hour and BsiWI in a 55°C water bath for 1 hour. The digested products were subjected to agarose gel electrophoresis (1.2% agarose gel). Positive bands were cut out under ultraviolet light, and gel recovery was carried out according to the procedures in the kit instructions (QIAquick Gel Extraction Kit, purchased from QIAGEN, USA).
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com