Indole andrographolide, ramification of indole andrographolide and preparing method and medical application of ramification
A technology of andrographolide and its derivatives, applied in chemical instruments and methods, drug combinations, antineoplastic drugs, etc., can solve the problems of disappearance, activity decline, unstable physical and chemical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Preparation of 14,19-O-bis(tert-butyldimethylsilyl)-andrographolide (2):
[0083] 14-O-tert-butyldimethylsilyl-andrographolide (1.37g, 2.95mmol) was dissolved in 10ml DMF, TBSCl (0.89g, 5.90mmol) and imidazole (0.24g, 3.54mmol) were dissolved in 10ml DMF , under an ice bath, slowly add the reaction solution dropwise, react for 20min after the dropwise addition, add EA (60ml) to dilute after the reaction, wash with an aqueous solution containing sodium bicarbonate (3x60ml), wash with a large amount of water (3x60ml), and wash with saturated brine ( 3x60ml), the organic layer was dried over anhydrous sodium sulfate. The dried organic layer was concentrated and purified by column chromatography (PE:EA=6:1). Finally, dry white solid 14,19-O-di(tert-butyldimethylsilyl)-andrographolide (1.09g, 1.88mmol, yield 76%) was obtained, and 14-O-tert-butyldimethylsilyl was recovered Silyl-andrographolide (0.22 g, 0.47 mmol). MP: 110-112°C. 1 H-NMR (300MHz, CDCl 3, ppm) δ: 6.83(t,...
Embodiment 2
[0085] Preparation of 14,19-O-di(tert-butyldimethylsilyl)-3-oxo-andrographolide (3):
[0086] Dissolve 14,19-O-bis(tert-butyldimethylsilyl)-andrographolide (0.60g, 1.04mmol) in 14ml of dichloromethane, stir at room temperature, add PCC (0.45g, 2.07mmol) And sodium acetate (0.34g, 4.15mmol), finally add 0.45g of silica gel, and react at room temperature. Stop the reaction after 24h, add silica gel to filter, concentrate the filtrate, and purify by column chromatography (PE:EA=5:1) to obtain a transparent viscous liquid, which turns into a white solid 14,19-O-di(tert-butyldi Methylsilyl)-3-oxo-andrographolide (0.53 g, 0.92 mmol, 88% yield). Mp: 90-92°C.1H-NMR (300MHz, CDCl 3 , ppm) δ: 6.84(t, 1H, J=5.7Hz), 5.06-5.08(m, 1H), 4.94(s, 1H), 4.65(s, 1H), 4.43(dd, 1H, J=6.5Hz , 9.8Hz), 4.10(dd, 1H, J=3.1Hz, 9.8Hz), 3.87(d, 1H, J=9.9Hz), 3.54(d, 1H, J=9.9Hz), 2.61-3.64(m, 2H), 1.97-2.02(m, 3H), 1.70-1.80(m, 1H), 1.59-1.64(m, 3H), 1.12(s, 3H), 1.00(s, 3H), 0.93(s, 9H) , 0.85(s, 9H)...
Embodiment 3
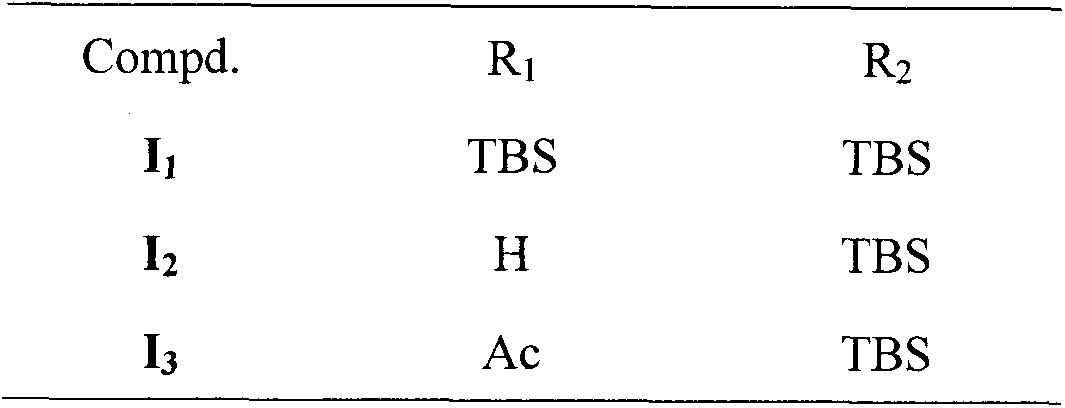
[0088] 14,19-O-bis(tert-butyldimethylsilyl)-indolo[3,2-b]andrographolide (I 1 ), 14-O-tert-butyldimethylsilyl-indolo[3,2-b]andrographolide (I 2 ) and 14-O-tert-butyldimethylsilyl-19-O-acetyl-indolo[3,2-b]andrographolide (I 3 ) preparation:
[0089] Add phenylhydrazine hydrochloride (0.60g, 4.15mmol) into the reaction flask, dissolve in 5ml of acetic acid, and dissolve 14,19-O-di(tert-butyldimethylsilyl)-3-oxo-andrographolide in 22ml Add acetic acid to the dropping funnel, N 2 Protection, reflux oil bath at 90-100°C, slowly add F to the reaction solution dropwise, stop the reaction after 3.5h, add the reaction solution to ice water, extract with EA (3x50ml), wash with saturated sodium bicarbonate (3x60ml), and finally saturated with salt Wash with water (3x60ml), dry over anhydrous sodium sulfate, concentrate the dry organic layer, and purify by column chromatography (PE:EA=6:1) to give yellow solid 14,19-O-bis(tert-butyldimethylsilyl) -indolo[3,2-b]andrographolide (I 1 ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com