Furan dioctyl phthalate-lactic acid-dihydric alcohol copolymer and preparation method thereof
A furandicarboxylic acid and diol technology, which is applied in the field of furandicarboxylic acid-lactic acid-diol copolymer and its preparation, can solve the problems of insignificant improvement in performance, reduced carboxylic acid activity, unfavorable copolyester synthesis, and the like, Achieve the effect of improving thermal and mechanical properties, good thermal and mechanical properties, and reducing utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
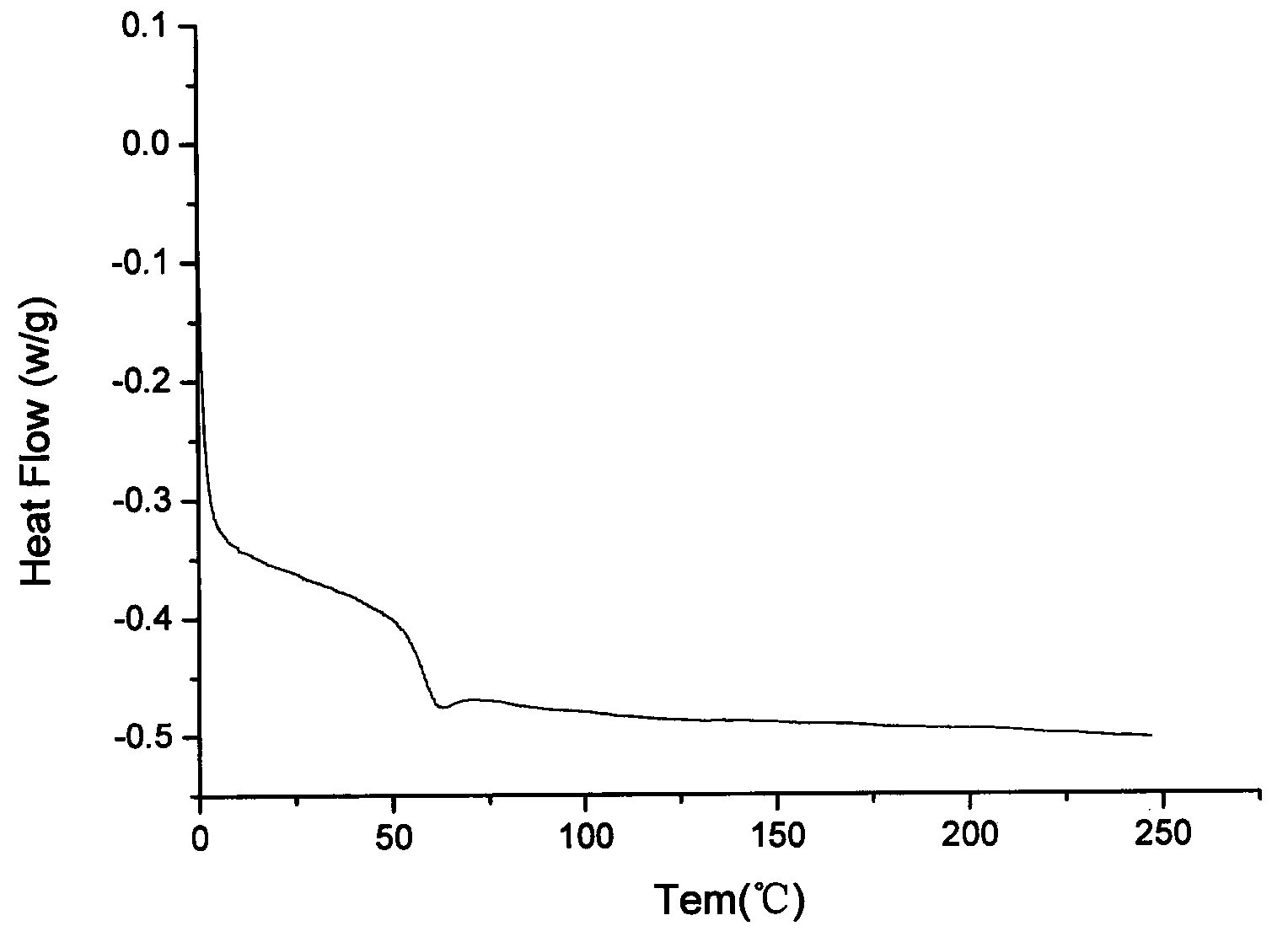
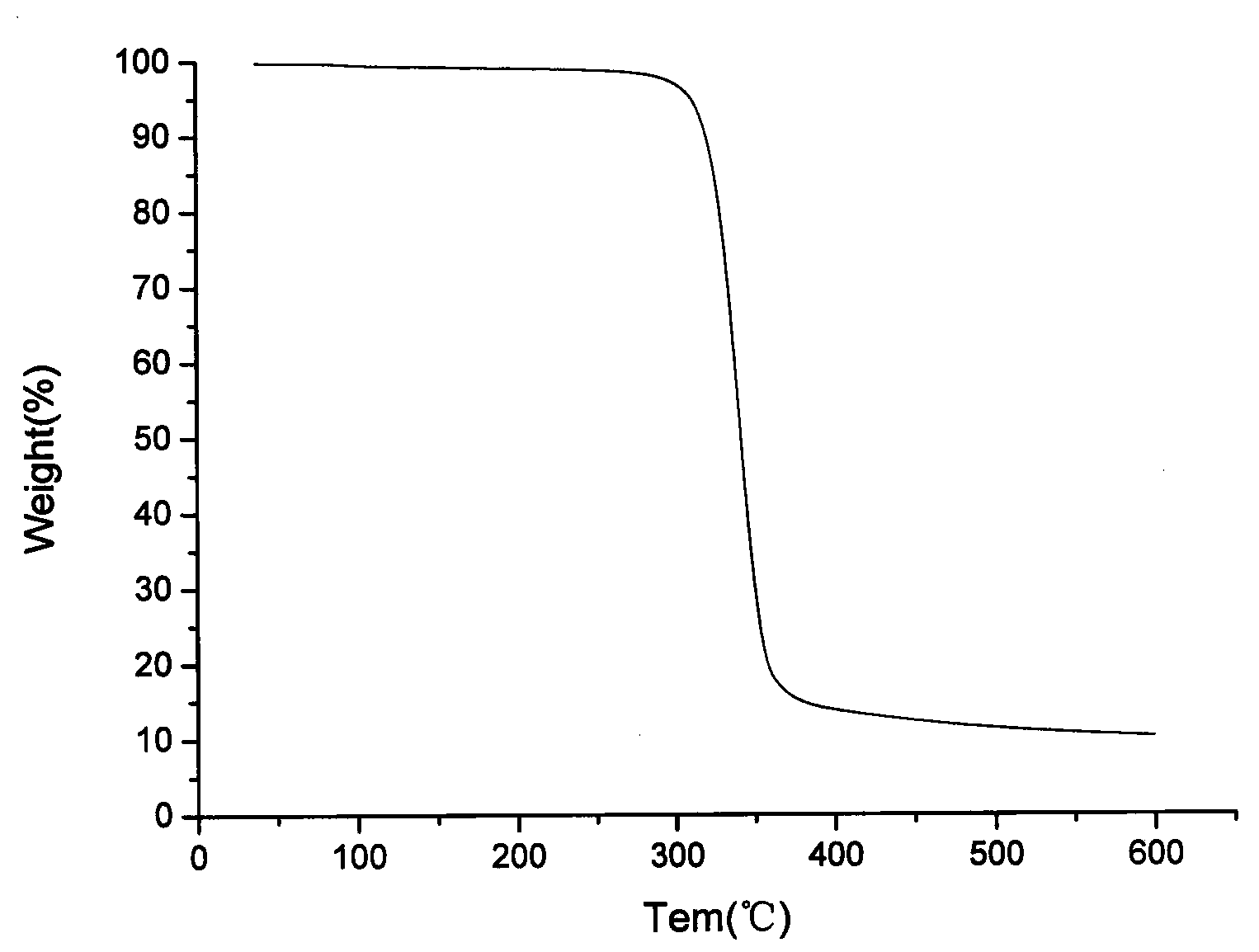
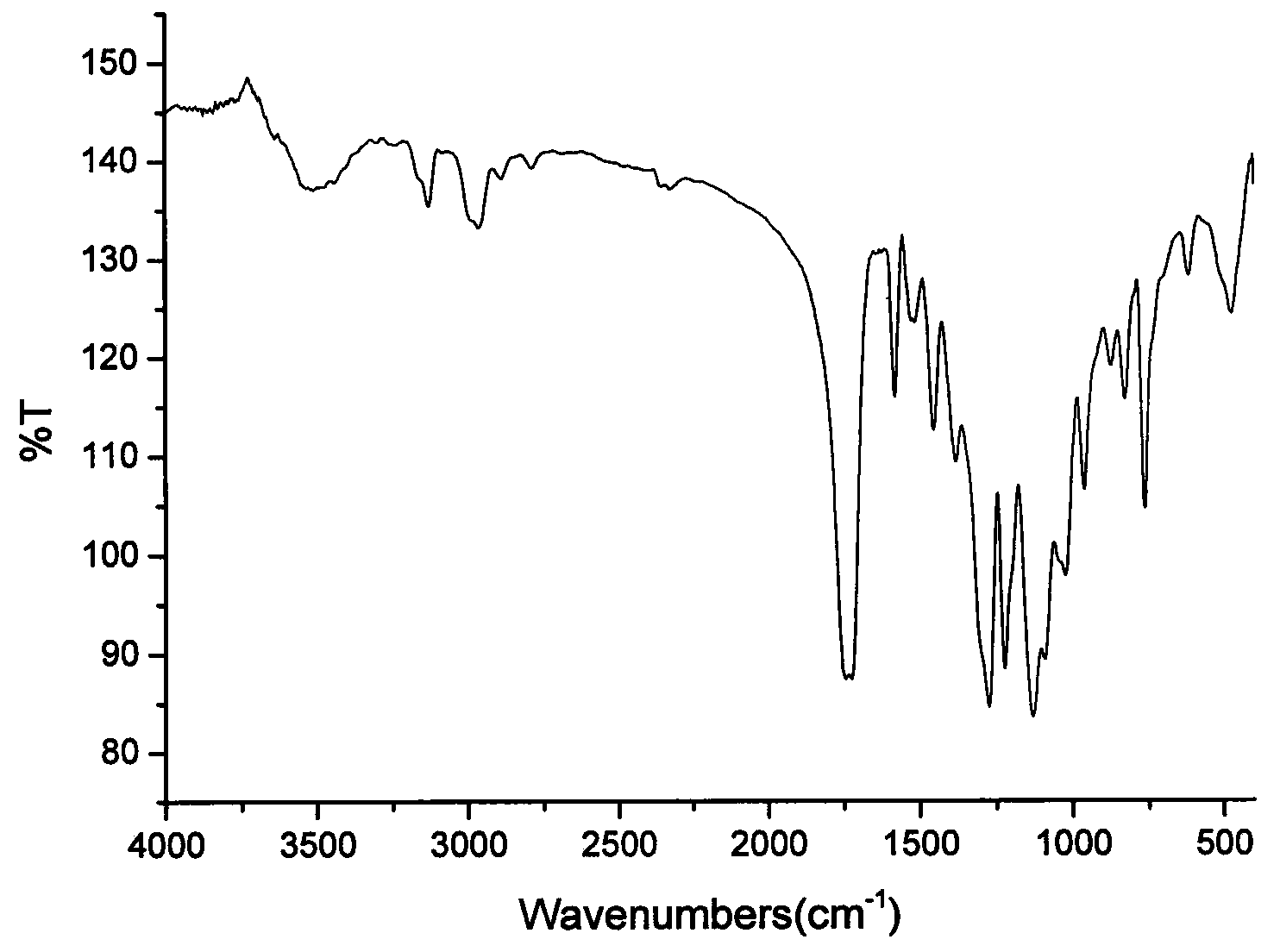
Image
Examples
Embodiment 1
[0028] Add 500g of 90% (mass fraction) L-lactic acid aqueous solution into a four-necked flask, heat it to 110°C for 2 hours under the protection of nitrogen at normal pressure, and then raise the temperature to 160°C for 2 hours, then keep this temperature and reduce pressure, at 100Pa The reaction was continued for 2 h to obtain oligomeric lactic acid (OLLA).
[0029] Furandicarboxylic acid (93.6g), ethylene glycol (94.5g), and tetrabutyl titanate (0.468g) were used as catalysts. In a nitrogen atmosphere, the reaction was mechanically stirred at 150°C under normal pressure for 5h until the solution became homogeneous and transparent. , generate dihydroxyethylene furandicarboxylate, then under nitrogen protection, add 265.8g OLLA and 0.28g trimethyl phosphate to the dihydroxyethylene furandicarboxylate of generation, seal, vacuumize to 500Pa, at 160 React at ℃ for 5 hours. After the reaction, dissolve the obtained product in dichloromethane, and then pour it into methanol. Th...
Embodiment 2
[0031] Add 400g of 80% (mass fraction) D-lactic acid aqueous solution into a four-necked flask, heat it to 120°C for 2 hours under the protection of nitrogen at normal pressure, then raise the temperature to 170°C for 3 hours, then keep this temperature and reduce the pressure, at 200Pa The reaction was continued for 4h to obtain oligomeric lactic acid (OLLA).
[0032] Furandicarboxylic acid (80g), 1,4-butanediol (79g), and tetraisopropyl titanate (0.68g) were used as catalysts. In a nitrogen atmosphere, the reaction was mechanically stirred at 170°C under normal pressure for 5h until the solution became Homogeneous and transparent, generate dihydroxybutylene furandicarboxylate, then under the protection of nitrogen, add 44.5g OLLA and 0.28g triethyl phosphate to the generated dihydroxybutylene furandicarboxylate, seal, and evacuate to 100Pa , react at 180°C for 4h, after the reaction, dissolve the obtained product in dichloromethane, then pour into methanol, the amount of met...
Embodiment 3
[0034] Add 300g of 85% (mass fraction) D, L-lactic acid aqueous solution into a four-necked flask, heat it to 130°C for 2h under normal pressure under the protection of nitrogen, then raise the temperature to 180°C for 3h, then keep the temperature and reduce the pressure. The reaction was continued for 6h at 300Pa to obtain oligomeric lactic acid (OLLA).
[0035]Furandicarboxylic acid (60g), 1,5-pentanediol (70g), and titanium tetrachloride (0.002g) were used as catalysts, and mechanically stirred at 180°C under normal pressure for 6 hours in a nitrogen atmosphere until the solution became homogeneous Transparent, generate dihydroxypentyl furandicarboxylate, then under the protection of nitrogen, add 155g OLLA and 0.8g triphenyl phosphite to the generated dihydroxypentyl furandicarboxylate, seal, vacuumize to 300Pa, in React at 195°C for 1 hour. After the reaction, dissolve the obtained product in dichloromethane, and then pour it into methanol. The amount of methanol added i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com