A kind of amino acid tanshinol ester derivative and its preparation method
A technology of tanshinol ester and amino acid, which is applied in the field of amino acid tanshinol ester derivatives and its preparation, can solve the problems of increasing water solubility and not meeting the requirements of clinical medication, and achieve the effect of good water solubility and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
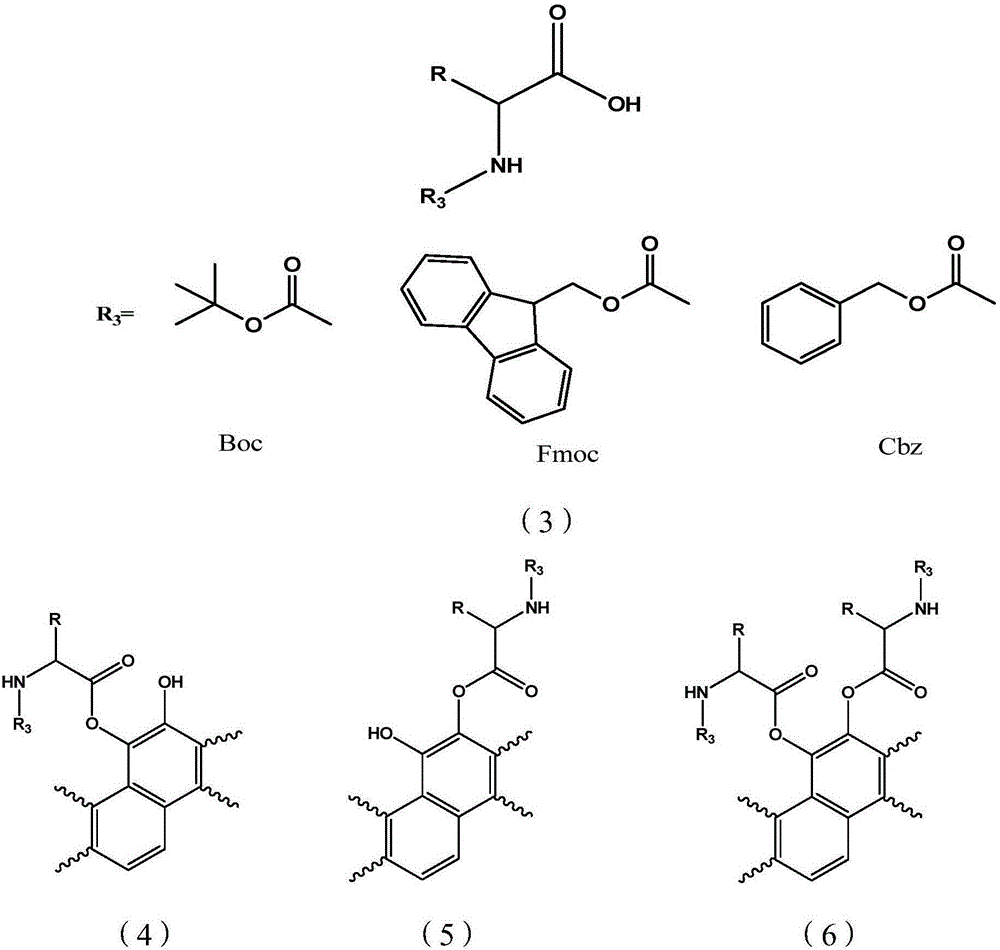
[0034] a: Reduction reaction: Weigh 2.94g of tanshinone IIA raw material into a 100ml three-necked bottle, add about 160mg of 10% palladium carbon (Pd / C), dissolve it in about 30ml of anhydrous THF, stir at room temperature under the protection of hydrogen until the color of the solution fades .
[0035] b: Preparation of amino acid intermediate: in a 500ml reaction flask, add 1.8g sodium bicarbonate, 180ml water, add 1.7g glycine under stirring in an ice bath, dissolve 3.8g benzyl chloroformate in 40ml ether, then add dropwise to In the amino acid reaction bottle, after the addition, slowly rise to room temperature and stir for 4 hours, pour into ice water, adjust pH=1.5 with hydrochloric acid, stir at room temperature for 15 minutes, add 200ml ether for extraction three times, the ether layer is washed three times with saturated saline, and the organic layer Dry over anhydrous sodium sulfate, filter, and column chromatography to obtain N-benzyloxycarbonylglycine (Cbzglycine)...
Embodiment 2
[0044] a: Reduction reaction: Weigh 2.94g of tanshinone IIA raw material into a 100ml three-necked bottle, add about 160mg of 10% palladium carbon (Pd / C), dissolve it in about 30ml of anhydrous THF, stir at room temperature under the protection of hydrogen until the color of the solution fades .
[0045] b: Preparation of amino acid intermediates: Add 1.7g of glycine, 0.4g of sodium hydroxide, 10ml of water and 10ml of dioxane into a single-port bottle, cool to an ice bath, slowly add 1.1 equivalents of Boc anhydride dropwise, and slowly rise to React at room temperature for more than 6hrs. The excess anhydride was removed by extraction with ethyl acetate, the pH of the aqueous layer was adjusted to 3-4 with 2N HCl, extracted with EA, dried and evaporated to dryness to obtain Boc amino acid for use.
[0046] c: Add an appropriate amount of THF and 1.1 equivalents of N-methylmorpholine to b, add 1.05 equivalents of pivaloyl chloride at -10°C, insert a drying tube and stir at l...
Embodiment 3
[0054] a: Reduction reaction: Weigh 2.94g of tanshinone IIA raw material into a 100ml three-neck flask, add about 300mg of 5% Pd / C, dissolve in about 50ml of anhydrous ethyl acetate, stir at room temperature under the protection of hydrogen until the color of the solution fades.
[0055] b: Preparation of amino acid intermediates: Weigh 2.2g of L-alanine and dissolve it in 30ml of 10% Na 2 C0 3 In the solution, stir in an ice bath; weigh 5.7g of fluorenylmethoxycarbonyl chloride (Fmoc-Cl) and dissolve it in 20ml of acetone, add it dropwise to the alanine solution system, after the addition is complete, stir in an ice bath for 30min, then stir at room temperature for 2.0 h. After the reaction is complete, pour it into 400ml of water, extract it twice with diethyl ether, cool it and adjust the pH to about 2 with concentrated hydrochloric acid, a large amount of white solid precipitates, extract it three times with 50ml ethyl acetate, dry it with anhydrous magnesium sulfate, rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com