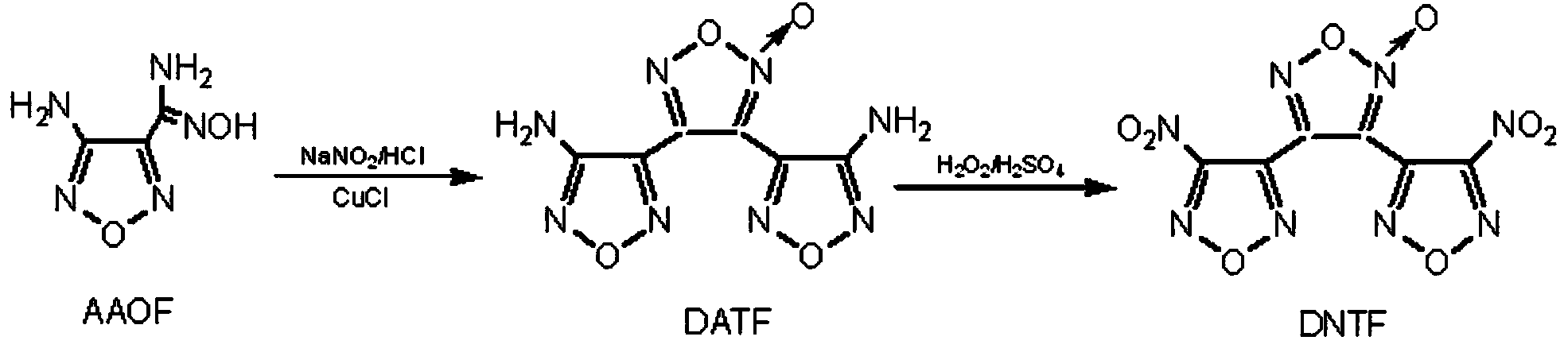
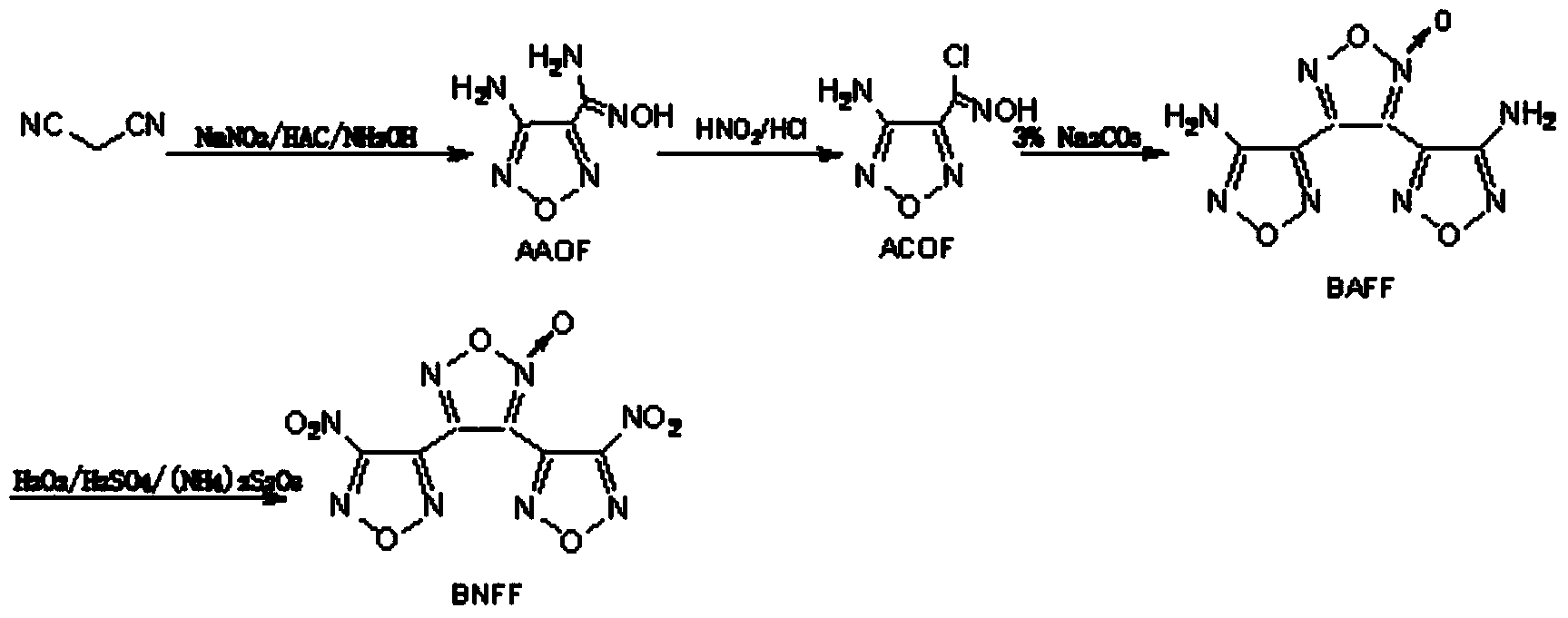
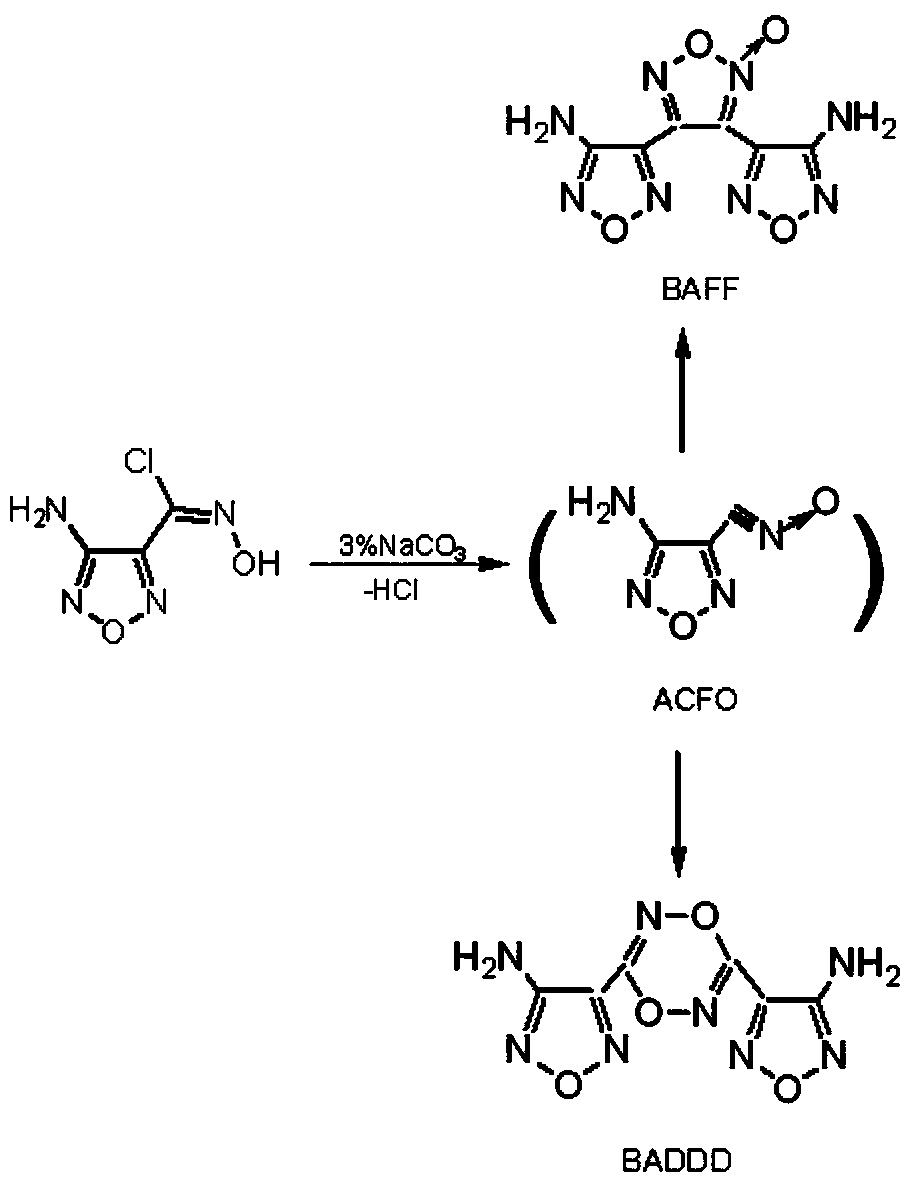
Preparation method of high-energy explosive 3,4-di(nitrofurazano)furoxan
A technology of nitrofurazan group and furoxan oxide is applied in directions such as organic chemistry, can solve problems such as incapability of engineering application, and achieves the effects of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of intermediate 3,4-bis(aminofurazanyl)furazan oxide
[0026] 7.96g (0.049mol) of 3-amino-4-chlorooximidofurazan was added to 98mL of tetrahydrofuran, the mixture was cooled to 0°C to 3°C, and under mechanical stirring, maintaining this temperature, 6.76g ( 0.0245 mol) silver carbonate. The mixture was reacted below 10°C for 3.0h. Filtration, collecting the filtrate, vacuum distillation to remove tetrahydrofuran, collecting the solid, drying to obtain 4.94g of 3,4-bis(aminofurazanyl)furazan oxide, yield 80%, white powdery solid after recrystallization from ethanol, melting point 167℃~168℃. Elemental Analysis: C 6 N 8 O 4 H 4 Calculated: C28.6; H1.6; N44.4; Found: C27.8; H1.3; N44.2. IR (KBr tablet, cm -1 ): 3455, 3321(-NH 2 ); 1638, 1027 (furazan ring); 1608, 1532, 1383, 1004 (oxyfurazan ring). MS(EI): m / z252 (M + ), 236(M-NH 2 ), 222(M-NO), 58(NH 2 -C=N-O), 30 (NO). 1 H NMR (DMSO, 300MHz): δ 6.374 (2H, -NH 2 )δ6.472(2H,-NH 2 )ppm. 13 C ...
Embodiment 2
[0032] (1) Preparation of intermediate 3,4-bis(aminofurazanyl)furazan oxide
[0033] Add 15.92g (0.098mol) of 3-amino-4-chlorooximidofurazan to 200mL of acetonitrile, cool the mixture to 0℃~3℃, keep this temperature under mechanical stirring, add 14.87g in small amounts in batches (0.054 mol) silver carbonate. The mixture was reacted below 10°C for 3.5h. Filtration, collecting the filtrate, vacuum distillation to remove acetonitrile, filtering, collecting the solid, drying to obtain 9.26g of 3,4-bis(aminofurazanyl)furazan oxide, yield 75%, melting point 167.5℃~168.2℃.
[0034] (2) Preparation of high-energy explosive BNFF
[0035] Under ice bath and stirring, add 50.0ml of hydrogen peroxide solution with a solute mass fraction of 50% in a three-necked flask, add 15.0ml of concentrated sulfuric acid with a solute mass fraction of 98%, and then add crude BAFF5.04g (0.02mol); Incubate the reaction below 10°C for 1.5h; then raise the temperature to 70°C and react for 3h. After...
Embodiment 3
[0039] (1) Preparation of intermediate 3,4-bis(aminofurazanyl)furazan oxide
[0040] Add 10.0 g (0.062 mol) of 3-amino-4-chlorooximofurazan to 125 mL of dimethylformamide (DMF), cool the mixture to 0°C to 3°C, maintain this temperature under mechanical stirring, and divide 10.26 g (0.037 mol) of silver carbonate were added in small portions. The mixture was reacted below 10°C for 4.0h. Filtration, collection of filtrate, vacuum distillation to remove DMF, filtration, collection of solids, drying to obtain 6.4 g of 3,4-bis(aminofurazanyl)furazan oxide, yield 82%, melting point 167.6°C~168.5°C.
[0041] (2) Preparation of high-energy explosive BNFF
[0042] Under ice bath and stirring, add 60.0ml of hydrogen peroxide solution with a solute mass fraction of 30% in a three-necked flask, add 20.0ml of concentrated sulfuric acid with a solute mass fraction of 98%, and then add crude BAFF5.04g (0.02mol); The reaction was kept at a temperature below 10°C for 1.5h; then the temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com