Method for preparing rare-earth magnetic ferrite through in-situ polymerization cracking process
A magnetic ferrite, in-situ polymerization technology, applied in the field of preparation of rare earth magnetic ferrite by in-situ polymerization cracking method, can solve the problems of difficult control of nanoparticle size distribution, poor structural stability, high preparation temperature, etc., and achieve nucleation and The effect of accelerated growth rate, increased diffusion ability, and low preparation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of rare earth magnetic ferrite PrFeO by an in-situ polymerization cracking method 3 Methods:
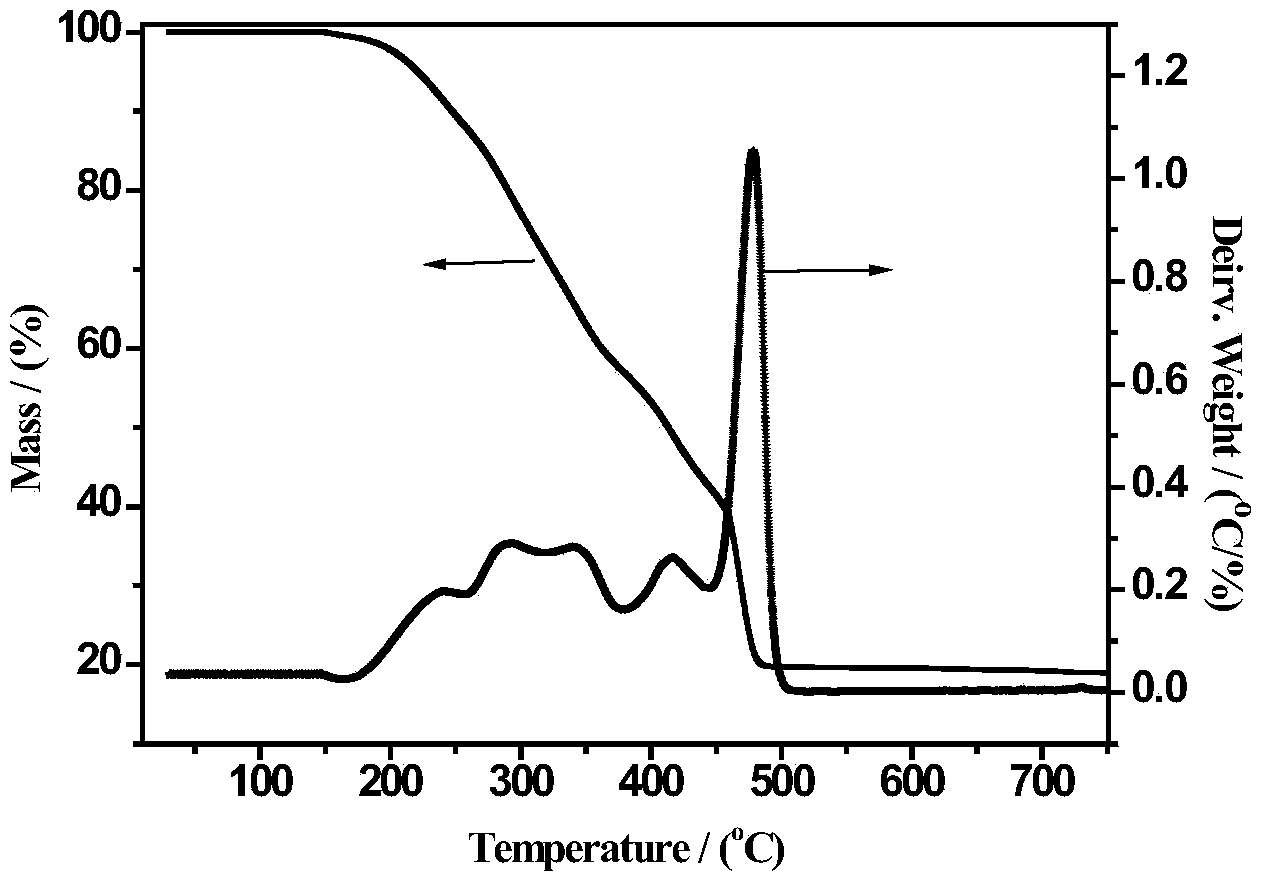
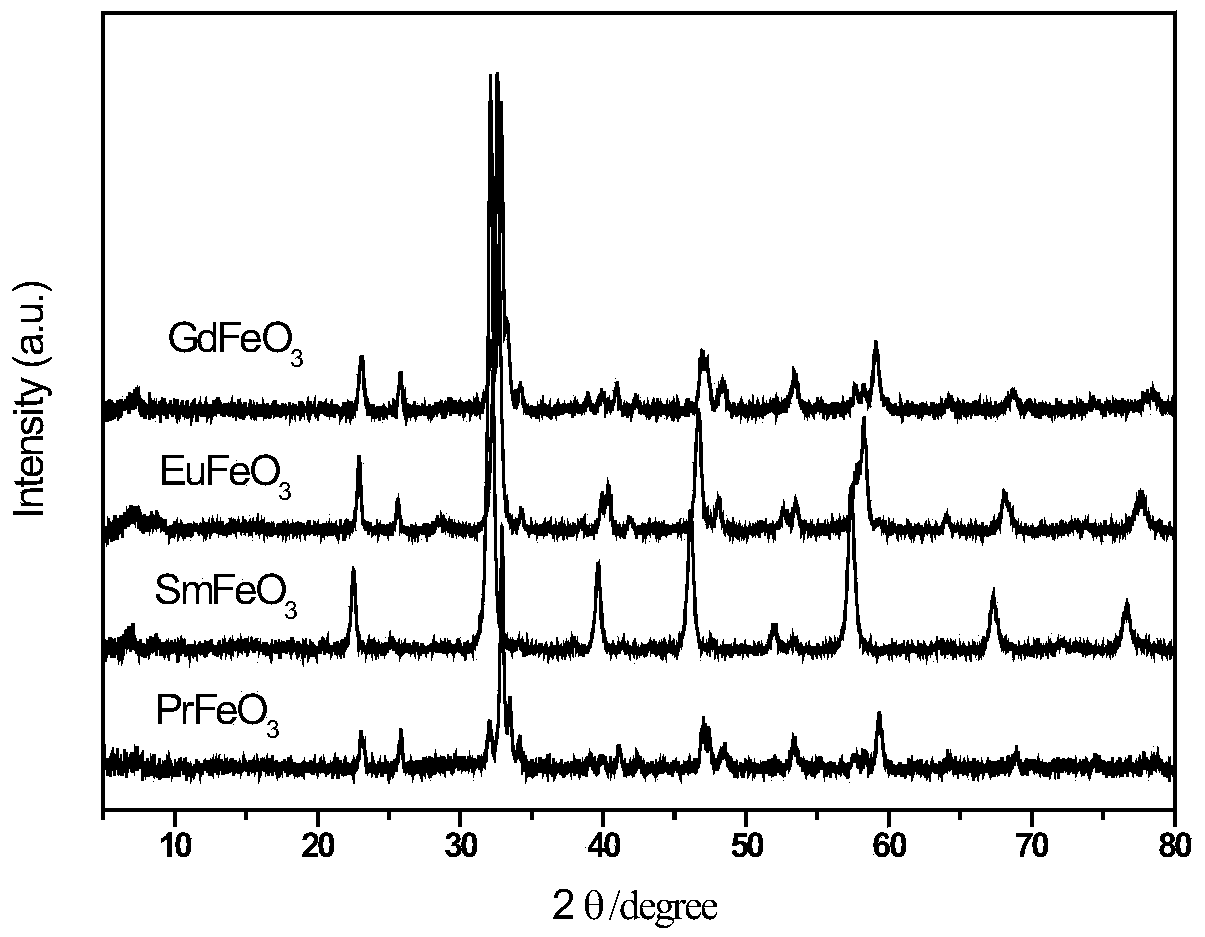
[0025] Fe(NO 3 ) 3 9H 2 O and Pr(NO 3 ) 3 ·6H 2 O was dissolved in an aqueous solution of acrylic acid and stirred well to form a transparent homogeneous solution. The mass ratio of the substance in the solution Fe(NO 3 ) 3 9H 2 O: acrylic acid: deionized water = 1:10:1. Then to the homogeneous solution, the concentration of 1 / 30 of the volume of acrylic acid was added to be 5 wt% (NH 4 ) 2 S 2 o 8 Aqueous solution was used as initiator, mixed evenly and then stirred at 80°C for 2 hours, fully polymerized to form a uniformly distributed polymer precursor, then dried at 120°C for 12 hours, and then crushed (thermogravimetric of the resulting product - difference see heat map figure 1 ), pyrolyzed at 500°C for 3 hours in an air atmosphere, and obtained a powder product after cooling to room temperature, which was confirmed to be PrFeO by characterizati...
Embodiment 2
[0027] Preparation of rare earth magnetic ferrite SmFeO by an in-situ polymerization cracking method 3 Methods:
[0028] Fe(NO 3 ) 3 9H 2 O and Sm(NO 3 ) 3 ·6H 2 O was dissolved in an aqueous solution of acrylic acid and stirred well to form a transparent homogeneous solution. The mass ratio of the substance in the solution Fe(NO 3 ) 3 9H 2 O: acrylic acid: deionized water = 1:10:1. Then to the homogeneous solution, the concentration of 1 / 30 of the volume of acrylic acid is 5wt% (NH 4 ) 2 S 2 o8 The aqueous solution is used as the initiator, mixed evenly and then stirred at 80°C for 2 hours to carry out sufficient polymerization reaction to form a uniformly distributed polymer precursor, then dried at 120°C for 12 hours, then crushed, and placed in an air atmosphere at 500°C After pyrolysis for 3 hours, the powder product was obtained after cooling to room temperature, which was confirmed to be SmFeO by characterization 3 .
Embodiment 3
[0030] Preparation of rare earth magnetic ferrite EuFeO by an in-situ polymerization cracking method 3 Methods:
[0031] Fe(NO 3 ) 3 9H 2 O and Eu(NO 3 ) 3 ·6H 2 O was dissolved in an aqueous solution of acrylic acid and stirred well to form a transparent homogeneous solution. The mass ratio of the substance in the solution Fe(NO 3 ) 3 9H 2 O: acrylic acid: deionized water = 1:10:1. Then to the homogeneous solution, the concentration of 1 / 30 of the volume of acrylic acid is 5wt% (NH 4 ) 2 S 2 o 8 The aqueous solution is used as the initiator, mixed evenly and then stirred at 80°C for 2 hours to carry out sufficient polymerization reaction to form a uniformly distributed polymer precursor, then dried at 120°C for 12 hours, then crushed, and placed in an air atmosphere at 500°C After pyrolysis for 3 hours, the powder product was obtained after cooling to room temperature, which was confirmed to be EuFeO by characterization 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com