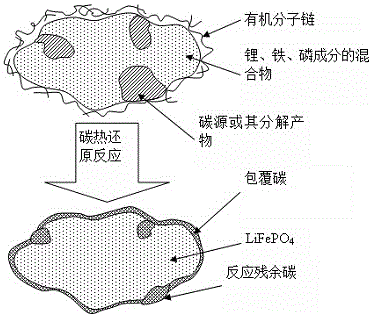
Method for preparing lithium iron phosphate electrode material based on two-time carbon addition process based on liquid phase mixing
A technology of lithium iron phosphate and electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as increased energy consumption, complicated process, and formation of a good carbon coating layer on the surface of lithium iron phosphate particles, so as to reduce production Effects of energy consumption, reduction of reaction temperature, improvement of batch stability and product performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
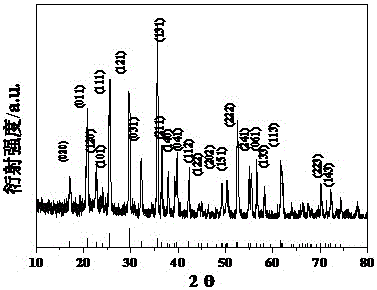
[0041] Weigh 1.208gLiCl·H with the molar ratio of lithium: iron: phosphorus = 1:1:1 2 O, 5.406 g FeCl 3 ·6H 2 0, 2.3006gNH 4 h 2 PO 4, and 0.35g of sucrose, which were dissolved in 20ml of deionized water, and then placed in an oven at a temperature of 195°C and heated in a slightly boiling state. After it was completely dried, the obtained solid residue was mixed with absolute ethanol dissolved in 0.35g PEG400, and ball milled for 5h. The anhydrous ethanol in the obtained ball-milled product was dried, and a high-temperature carbothermal reduction reaction was carried out in a tube furnace under the protection of flowing argon. The reaction temperature was 750° C. and the reaction time was 8 hours. like figure 2 As shown, the position of the XRD diffraction peak is related to that of LiFePO 4 The standard card (JCPDS Card No. 83-2092) matches, indicating that the obtained product is LiFePO 4 .
Embodiment 2
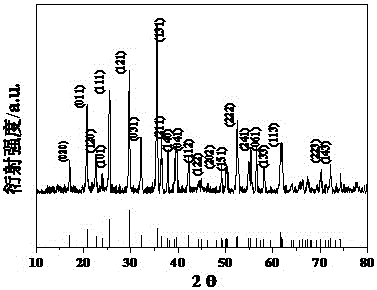
[0043] The preparation steps in this example are exactly the same as those in Example 1 above. The difference is: Weigh 1.379g anhydrous lithium nitrate, 8.08gFe(NO 3 ) 3 9H 2 O, 2.3006gNH 4 h 2 PO 4 , and 1.25 g of sucrose as a reactant. like image 3 As shown, the position of the XRD diffraction peak is related to that of LiFePO 4 The standard card (JCPDS Card No. 83-2092) matches, indicating that the obtained product is LiFePO 4 . Figure 4 Shown is the SEM picture of the material, and the particle size of the material can be seen in the figure to be several hundred nanometers. like Figure 5 As shown, the initial discharge capacity of the material is 156mAh / g at a charge-discharge rate of 0.1C, and the plateau voltage difference is about 0.15V. exist Image 6 Among them, the discharge capacity of the material at 0.1C gradually increases with the activation of the battery and the full infiltration of the electrolyte in the first few cycles, reaching 163 mAh / g at...
Embodiment 3
[0045] The preparation steps in this example are exactly the same as those in Example 2 above. The difference is: (1) The molar ratio of the raw materials added is lithium: iron: phosphorus = 1.05:1:1. (2) 0.45g of PEG400 is added when chain organic matter is added. like Figure 7 As shown, the material in Example 3 has a discharge capacity of 158 mAh / g at 0.1C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com