Tumor-treatment adenovirus vaccine taking hTERT (human telomerase reverse transcriptase) as target point
A technology for tumor treatment and recombinant adenovirus, which is applied in anti-tumor drugs, gene therapy, virus/bacteriophage, etc., and can solve the problem of low infection efficiency of blood-derived cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079]Example 1. Obtaining recombinant adenovirus co-expressing eTERTFc fusion antigen, GM / CSF and B7.1
[0080] 1. Construction of tumor therapeutic adenoviral vector Ad5F11p-eTERTFcGB
[0081] 1. Construction of recombinant shuttle vector pShuttle-CMV-eTERTFcGB
[0082] In the recombinant shuttle vector, the hTERT complex gene eTERTFcGB is composed of eTERTFc fusion antigen gene, GM / CSF gene (GenBank number: NM-000758) and B7.1 gene (GenBank number: NM-005191);
[0083] The eTERTFc fusion antigen gene consists of a human hTERT gene 1609-2628 base gene fragment (GenBank No.: NM_198253), a human IgG Fc segment gene (GenBank No.: Z17370) and a glycosylphosphatidylinositol (GPI) anchor peptide The composition of the gene (GenBank number: XM676434), and the nucleotide sequence of the eTERTFc fusion antigen gene is shown in sequence 1 in the sequence listing.
[0084] The eTERTFc fusion antigen gene is connected with the GM / CSF gene and the B7.1 gene through the IRES sequence, a...
Embodiment 2
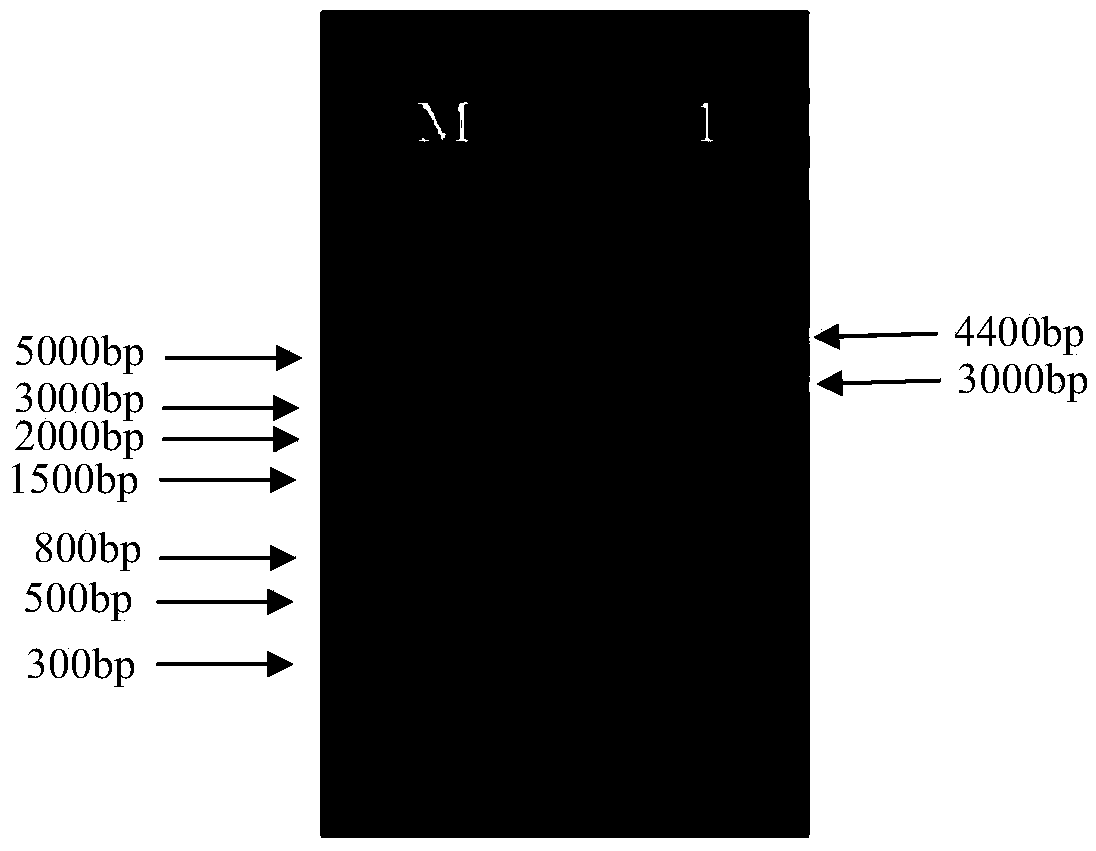
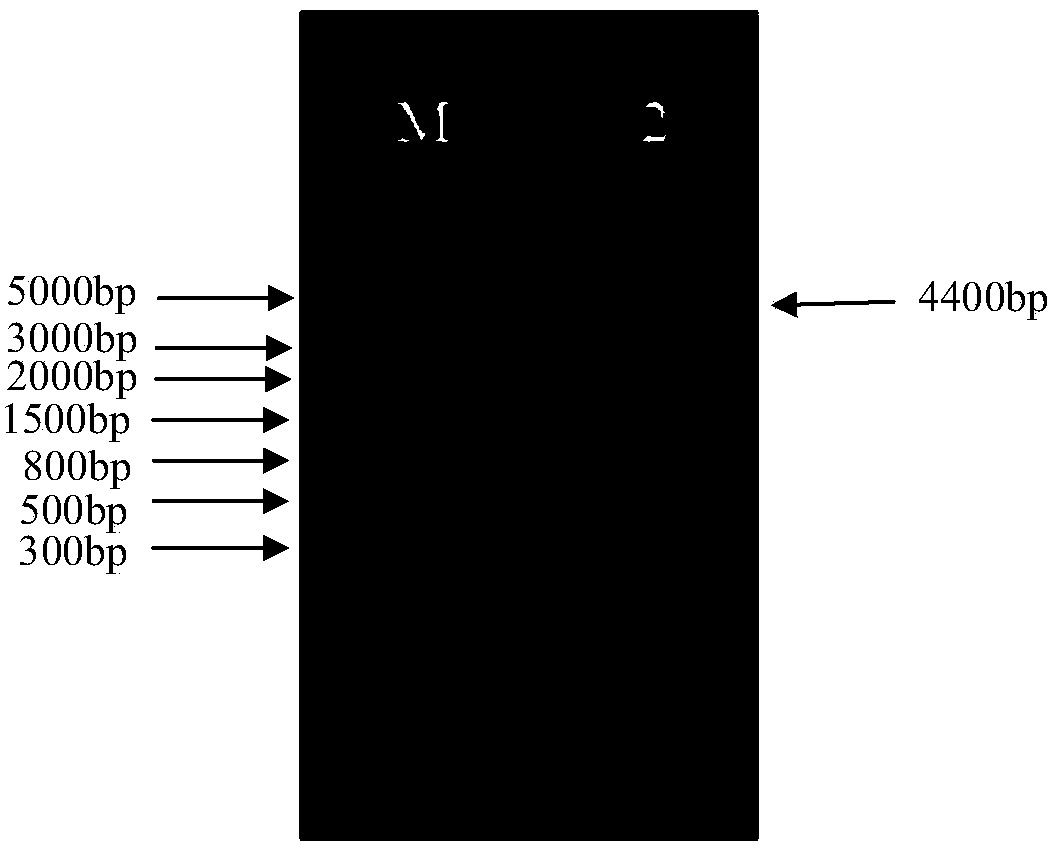
[0205] Example 2. Amplification, purification and titer determination of tumor therapeutic adenovirus vaccine targeting hTERT
[0206] In recent years, recombinant adenovirus vectors have been increasingly used in the field of gene therapy. In order to meet the needs of preclinical animal experiments and clinical human trials, a large number of recombinant adenoviruses must be produced and purified through an effective and flexible process. product. The traditional method is cyanogen chloride density gradient ultracentrifugation, which can produce a small amount of clinical-grade adenovirus products, but with the advent of the era of gene therapy, a large amount of adenovirus products are required. The "Q Sepharose XL virus licensed" developed by GE based on the ion exchange medium can provide a fast and scalable method for purification of adenovirus. The purpose of this example is to conduct experimental research on the amplification and purification process of the recombina...
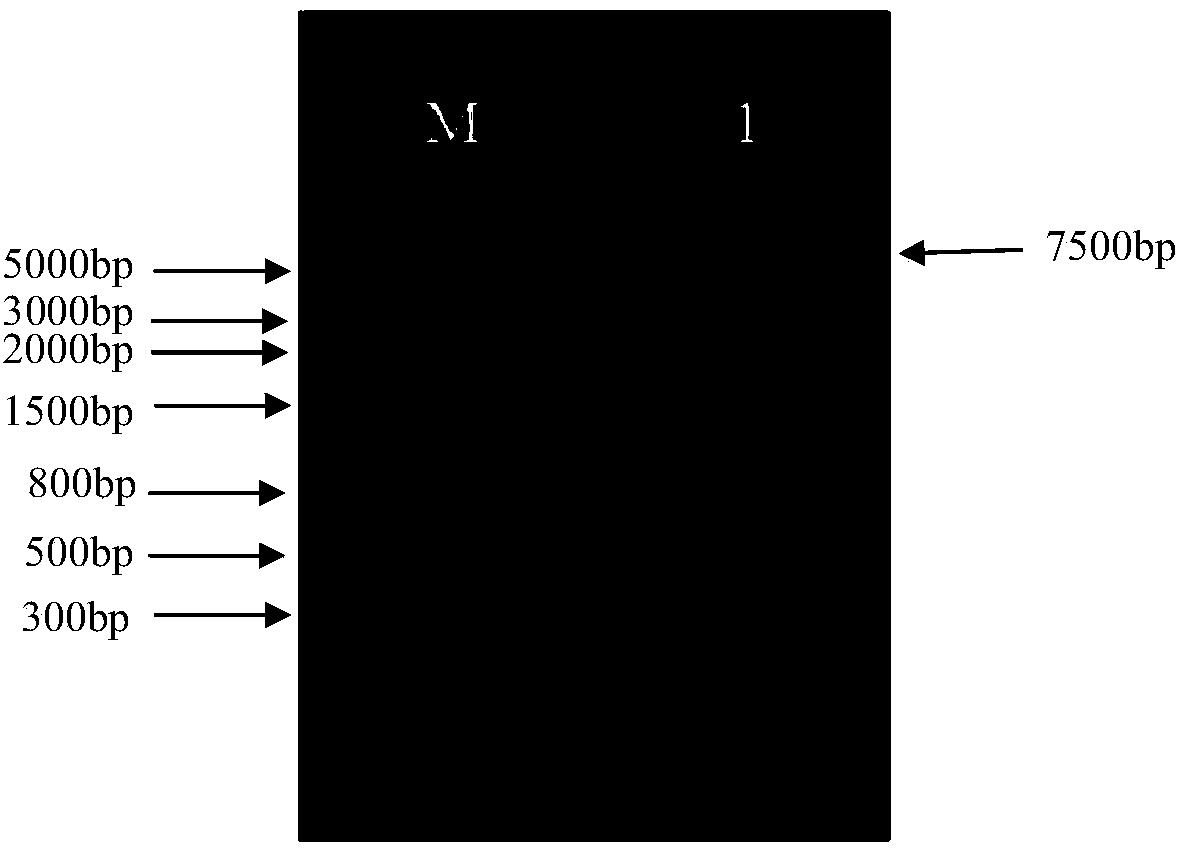
Embodiment 3
[0278] Example 3, Prokaryotic expression, purification and antigenic activity detection of telomerase reverse transcriptase hTERT antigenic fragment
[0279] At present, preclinical studies of broad-spectrum tumor immunotherapeutic vaccines targeting hTERT are mostly evaluated using mice or transgenic mouse tumor-bearing models. In order to further explore the effect of hTERT in immunotherapy of malignant tumors and its related mechanisms, The hTERT protein (1609-2628 bases) was purified and its immunogenicity was tested, which laid the foundation for the next step of the research on the immunological mechanism of Ad5F11p-eTERTFcGB in the tumor therapeutic adenovirus vaccine. .
[0280] First, the PCR method was used to amplify the hTERT1609-2628 base sequence, which was constructed into the prokaryotic expression vector pET-42a and induced to express in Escherichia coli E.coli.Rossata (DE3), and the expression conditions of hTERT protein were optimized Finally, determine the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com