Method for expressing cecropin AD by virtue of bacillus subtilis and preparation method of cecropin AD
A technology of Bacillus subtilis and cecropin, applied in the field of preparation of biological preparations, can solve the problems of low content of natural antibacterial peptides, high cost of chemical synthesis, difficulty in large-scale production, etc., and achieves reduced enzyme activity, safe, efficient, and easy price. Effects of Competence Conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method utilizing Bacillus subtilis to express cecropin AD, as follows:
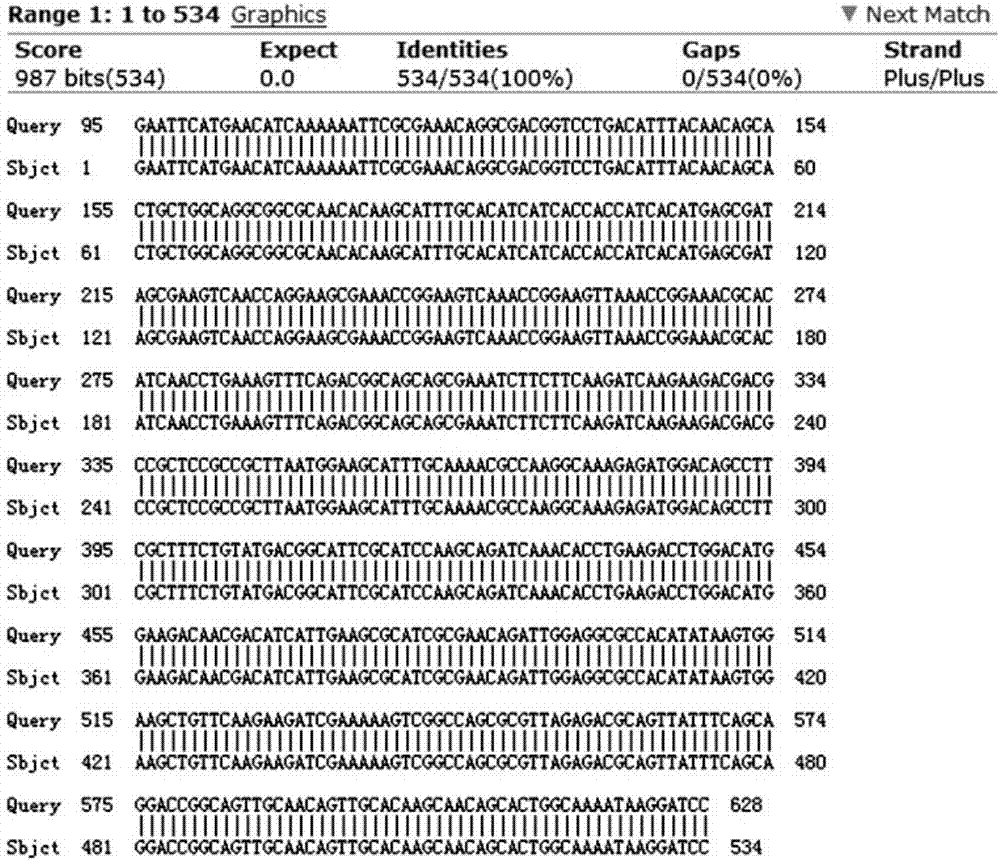
[0028] (1) Gene synthesis: artificially design the coding target gene fragment, fuse the signal peptide SPsacB gene, six histidine genes, SUMO tag upstream of the cecropin AD gene, and add an EcoRI restriction site at the 5' end. Add stop codon and BamHI restriction site at the 3' end, the entire fusion gene SPsacB-6×His-SUMO-Cecropin AD, its nucleotide sequence is shown in SEQ ID NO.1, and its amino acid sequence is shown in SEQ ID As shown in NO.2;
[0029] (2), construction and transformation of the expression plasmid: the expression vector uses the Escherichia coli-Bacillus subtilis shuttle vector pGJ148 as the backbone, the promoter is the maltose promoter Pglv, and the gene-deficient strain Bacillus subtilis WB800N is used as the expression host bacterium. Transformation method The recombinant vector is directly transformed into the expression host Bacillus subtilis WB800N, and the express...
Embodiment 2
[0032] A method utilizing Bacillus subtilis to prepare cecropin AD, as follows:
[0033] 1. Gene synthesis
[0034] The amino acid sequence of cecropin AD is: KWKLFKKIEK VGQRVRDAVI SAGPAVATVA QATALAK, the coding gene fragment is artificially designed, and an EcoRI restriction site and signal peptide SP are added at the 5' end sacB , 6×His tag, SUMO, plus a stop codon and a BamHI restriction site at the 3’ end, the entire fusion gene SPsacB-6×His-SUMO-Cecropin AD, its nucleotide sequence is as shown in SEQ ID NO.1 As shown, its amino acid sequence is shown in SEQ ID NO.2, and the gene synthesis was completed by Beijing Liuhe Huada Gene Technology Co., Ltd.
[0035] 2. Construction and transformation of expression plasmids
[0036] 1) Cloning the artificially synthesized coding gene into the Bacillus subtilis expression vector to construct a recombinant expression vector, and the constructed recombinant expression vector is positively screened by single and double enzyme diges...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com