Recombinant expression of human desmocyte growth factor-21
A growth factor and expression carrier technology, applied in the field of recombinant expression of human fibroblast growth factor-21, can solve the problems of affecting protein activity, difficulties in medical research and drug development, and low product recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] PCR synthesis of embodiment 1 FGF-21 gene
[0038] According to the amino acid sequence of FGF-21 gene with signal peptide removed, primers were designed, and the FGF-21 gene with signal peptide removed was obtained by complementary extension. According to the degeneracy of codons, the enzyme cutting sites used in this experiment were eliminated, and the online software Primer Finder and DNA STAR were used to design FGF-21 primers, a total of 14 (see the sequence listing for its nucleotide sequence), every two There is a 20bp complementary sequence between the strips, and the N-terminal primer contains a complementary sequence to the Sumo molecular chaperone. The FGF-21 gene was synthesized by bridge PCR.
[0039] FGF-21 gene synthesis
[0040] PCR system one:
[0041] 10×pfu buffer 10μl
[0042] dNTP (2.5mM) 8μl
[0043] Primer F1 (10μmol) 2μl
[0044] Primer R1 (10μmol) 2μl
[0045] pfu DNA polymerase 0.5μl
[0046] ddH2O 77.5μl
[0047] Total 100μl
[004...
Embodiment 2
[0066] Example 2 Construction of pET-20b-Sumo-FGF-21 expression vector
[0067] In order to fuse the synthetic FGF-21 gene with the Sumo molecular chaperone, the synthetic primer P1 was designed according to the N-terminal of the Sumo sequence, and the primer P2 was designed according to the C-terminal of the FGF-21 gene. In the reaction system containing the FGF-21 gene and the Sumo molecular chaperone, Using P1 and P2 as primers, the fusion gene containing Sumo-FGF-21 was amplified by PCR. Then the fragment was double-digested with NdeI and XhoI, and ligated with the pET-20b plasmid that was also double-digested with the two enzymes.
[0068] Nucleotide sequences of primers P1 and P2:
[0069] P1: 5'GGAATTCCATATGCATCATCATCATCATCATCACG 3'
[0070] P2: 5' CCGCTCGAG TCAGGAAGCGTAGCTGGGGCT 3'
[0071] PCR reaction system:
[0072] 10×pfu buffer 2μl
[0073] dNTP (2.5mM) 2μl
[0074] Primer P1 (10μmol) 1μl
[0075] Primer P2 (10μmol) 1μl
[0076] FGF21 sequence: 0.5μl
[...
Embodiment 3
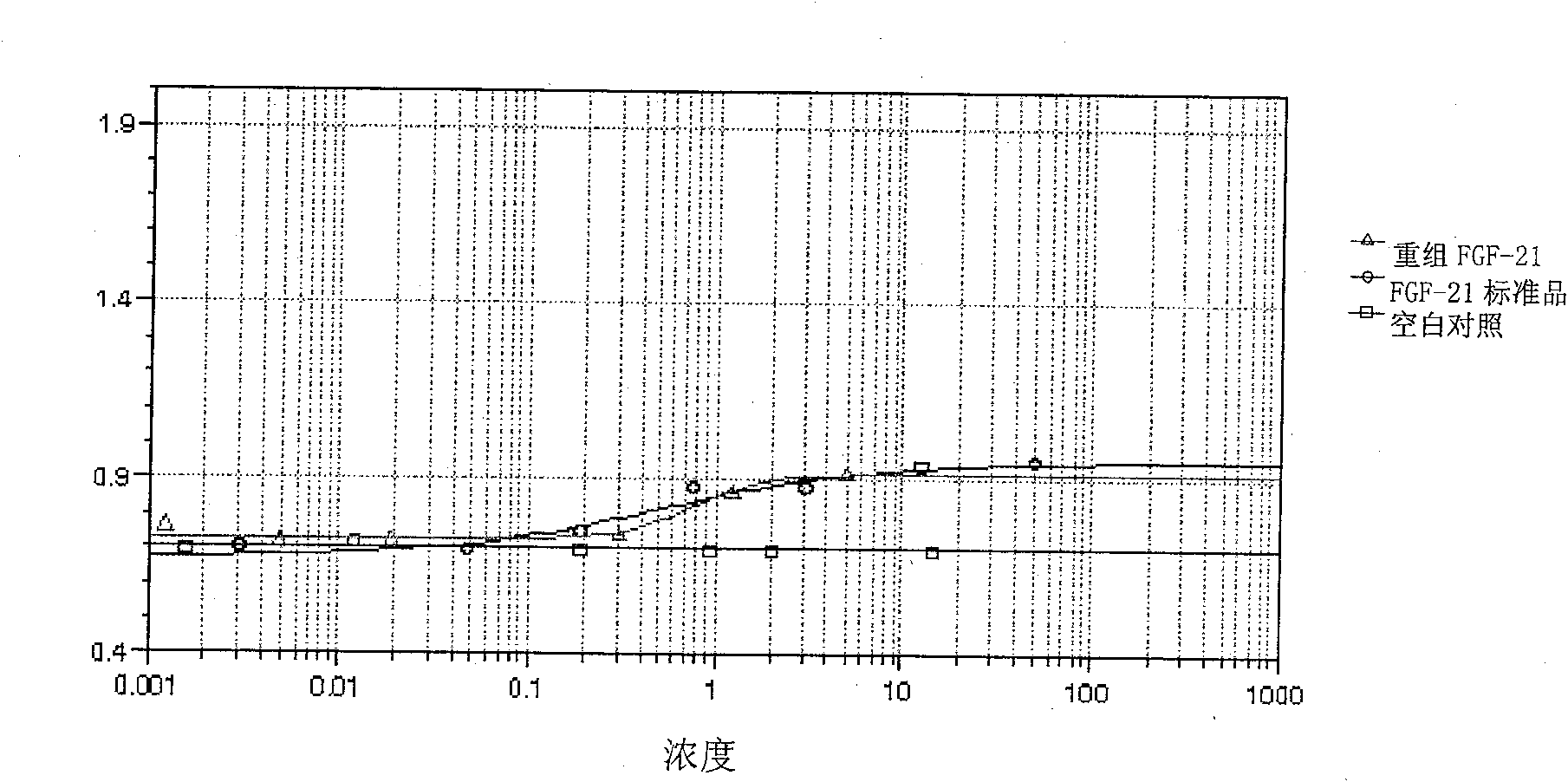
[0082] Embodiment 3: the acquisition of recombinant FGF-21 protein
[0083] 1. Induced expression
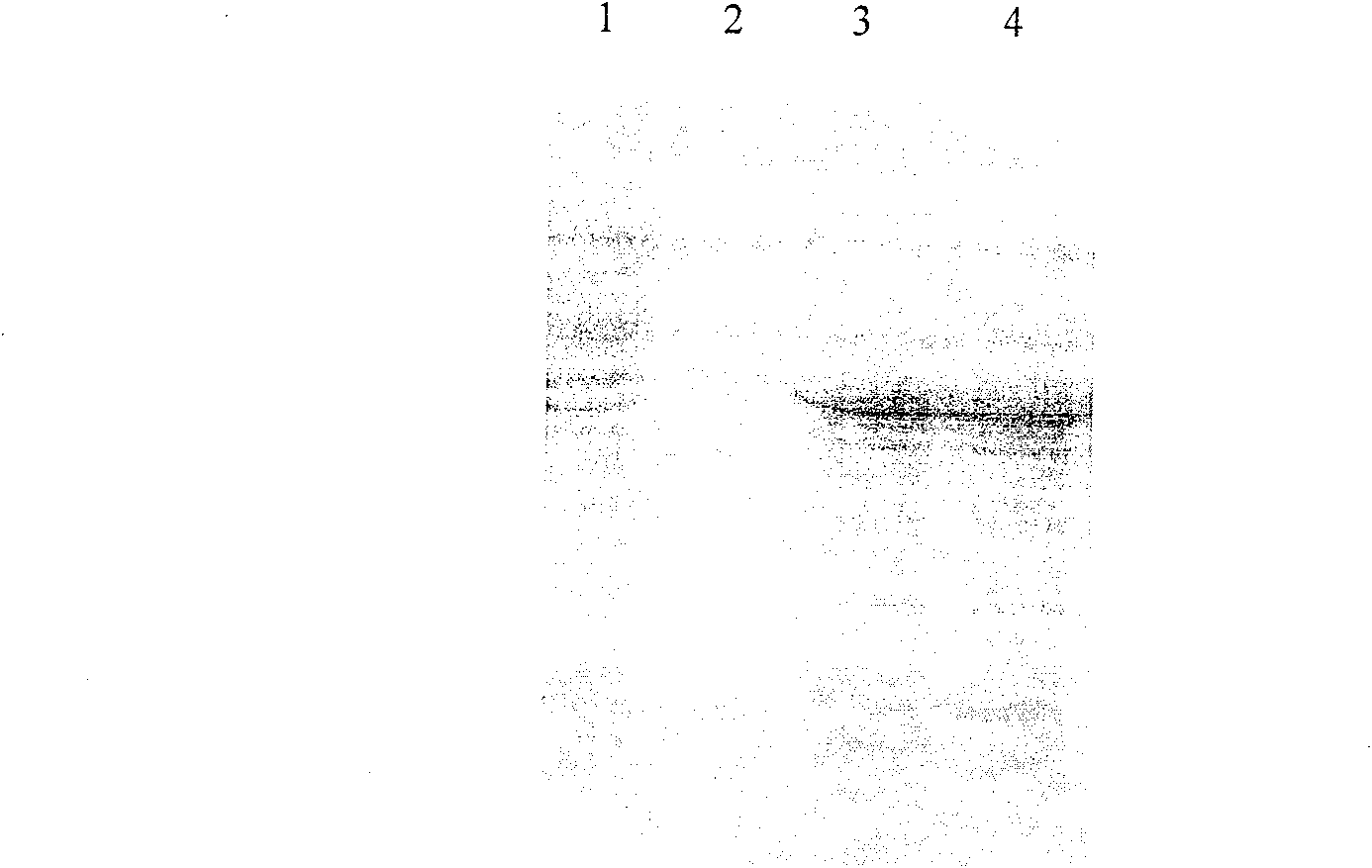
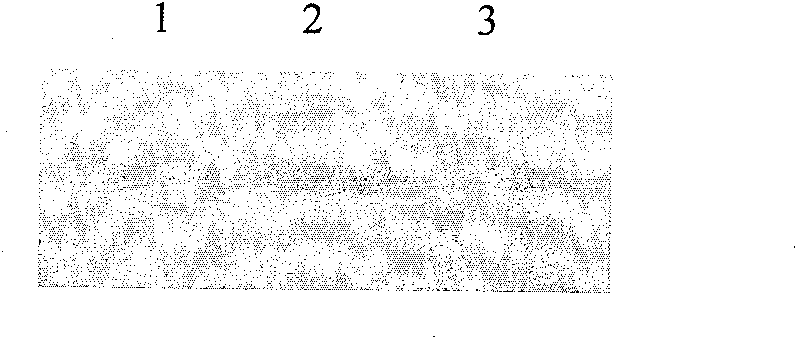
[0084]The correctly sequenced pET-20b-Sumo-FGF21 was transformed into Escherichia coli BL21(DE3) competent cells, and positive transformants were screened on an ampicillin resistance plate. A single colony of the screened positive recombinant bacteria BL21(DE3) / pET-20b-Sumo-FGF-21 was inoculated into LB medium and cultured with shaking at 37°C for 16h. Then it was transferred to fresh LB medium at a ratio of 5%, and the expression conditions such as temperature, IPTG concentration and induction time were optimized. Studies have confirmed that when the OD value is 0.6, 1mM IPTG is induced at 37°C for 3 hours, ultrasonic (100w, ultrasonic 2s, interval 2s) breaks the bacteria, and the soluble expressed target protein can account for more than 90% of the total bacterial target protein.
[0085] 2. Protein Purification
[0086] According to the above-mentioned optimal conditions f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com