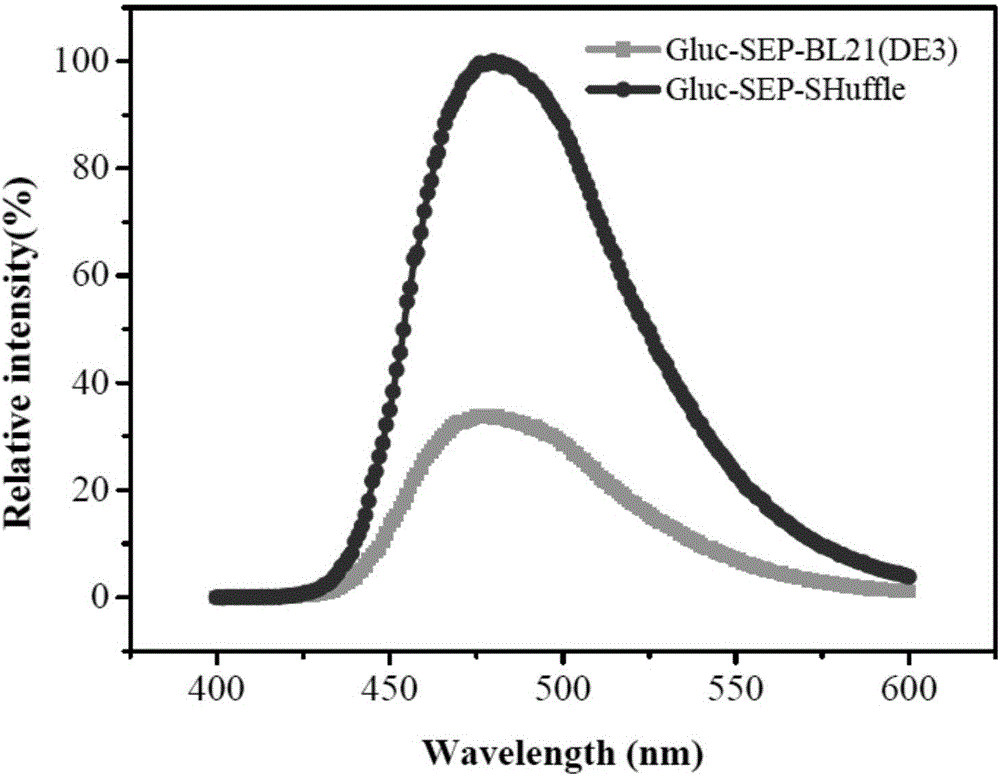
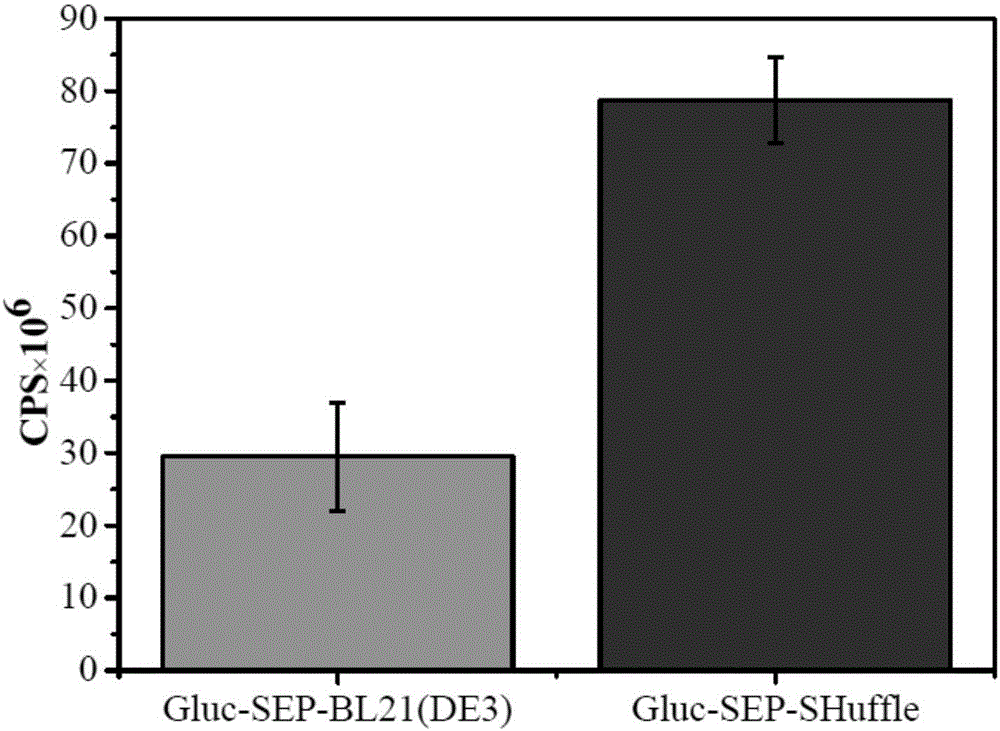
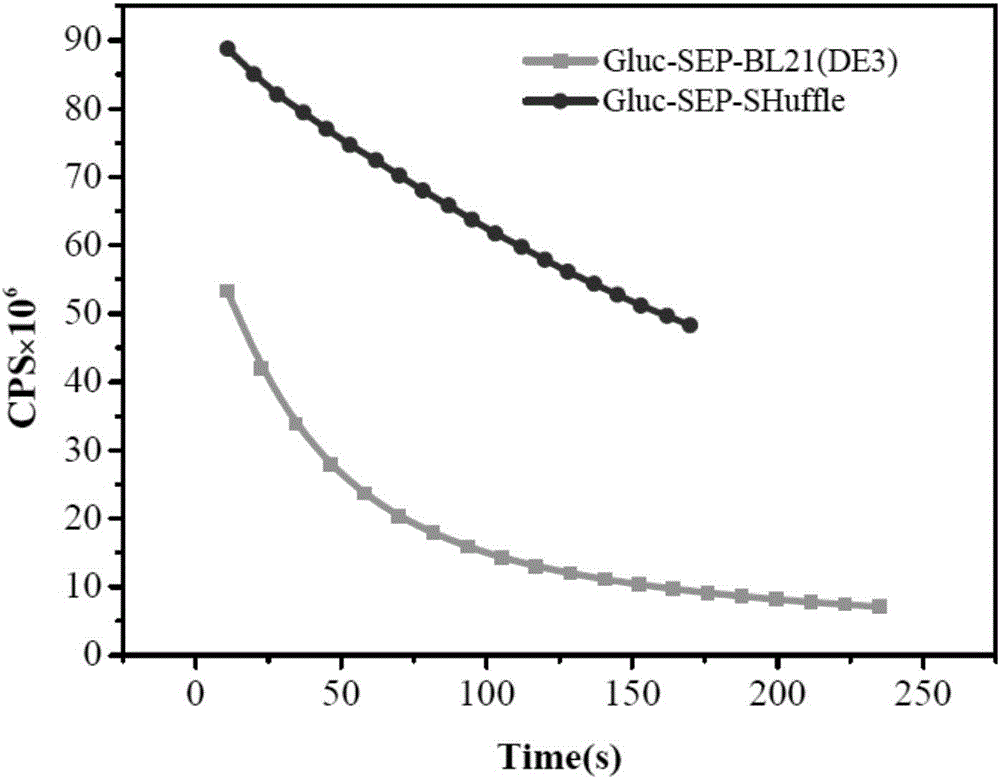
Disulfide bond protein based on Gluc luciferase and preparation method
A technology of luciferase and luciferase gene, applied in the field of disulfide bond protein and preparation based on Gluc luciferase, which can solve the problems of rapid fluorescence quenching, restriction of disulfide bond correct folding, poor optical stability of luciferase, etc. problem, to achieve the effect of strong fluorescence activity and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Construction of Luciferase Gluc-SEP Gene Expression Vector
[0041] 1. Primers
[0042] Forward primer:
[0043] 5'-CGCGGATCCATGAAGCCCACCGAGAACAACGAA-3'; (SEQ ID NO: 1)
[0044] Reverse primer:
[0045]5'-ACGCCGTCGACGTCGTCGTCTCCGTCGTCGTCTCCGTCACCACCGGCCCCCTTGATCTT-3' (SEQ ID NO: 2)
[0046] 2. PCR amplification of Gluc luciferase gene
[0047] PCR reaction system:
[0048] 5μL 10×High Figelity PCR buffer, 15mM MgCl 2 , 1mM dNTP Mix, 0.3μM forward primer, 0.3μM reverse primer, 10pg template, 1.25U High Fidelity PCR Enzyme Mix, and finally add double distilled water to make the total system 50μL;
[0049] PCR reaction conditions:
[0050] Pre-denaturation at 94°C for 3min, denaturation at 94°C for 30s, annealing at 58°C for 30s, extension at 72°C for 40s, reaction cycle 25 times, and final extension at 72°C for 10min.
[0051] 3. Construction of luciferase Gluc-SEP gene expression vector
[0052] The PCR amplification product containing the Gluc luciferase gene o...
Embodiment 2
[0058] Preparation of Gluc-SEP-Shuffle expression strain
[0059] The luciferase Gluc-SEP gene expression vector of Example 1 was transformed into Escherichia coli competent cell DH5α by heat treatment at 42°C, recovered by culturing at 37°C and applied to LB plate (containing 25 μg / ml kanamycin) for screening, Colonies were picked for colony PCR and double enzyme digestion to identify positive clones. The positive strains screened were cultured, plasmids were extracted, and finally identified by gene sequencing; after that, the correct plasmids identified by sequencing were heat-treated and transformed into SHuffle T7B E.coli expression strains, and Glycerol bacteria were preserved and frozen for future use.
Embodiment 3
[0061] Expression and purification of Gluc-SEP-Shuffle protein
[0062] First, inoculate the frozen Glycerolbacterium Gluc-SEP-SHuffle into LB liquid medium (containing 25 μg / ml ampicillin), cultivate the activated strain overnight at 37°C and 225 rpm, and then inoculate the activated bacterial liquid at a ratio of 1:100 In fresh LB liquid medium (containing 100 μg / ml ampicillin), cultivate until OD600 reaches 0.4-0.6, which is the growth logarithmic phase of the strain, and then add the inducer isopropyl-β-D-1-sulfur The galactoside IPTG (final concentration: 1mM) induced protein expression at 37°C for 7h, centrifuged at 11000rpm at 4°C for 10min to collect the bacteria, discarded the supernatant LB medium, added a certain amount of binding buffer (20mM Na 3 PO 4 , 0.5M NaCl, 40mM imidazole solution, pH 7.4) suspend the bacteria, use ultrasonic to break the bacteria, ultrasonic 3sec, interval 4sec, ultrasonic until the solution is completely clear and transparent, then centr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com