Polymer, light response micelle, light response drug-loading micelle and preparation methods thereof
A technology of drug-loaded micelles and polymers, applied in the field of nanomedicine, can solve the problem of low controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
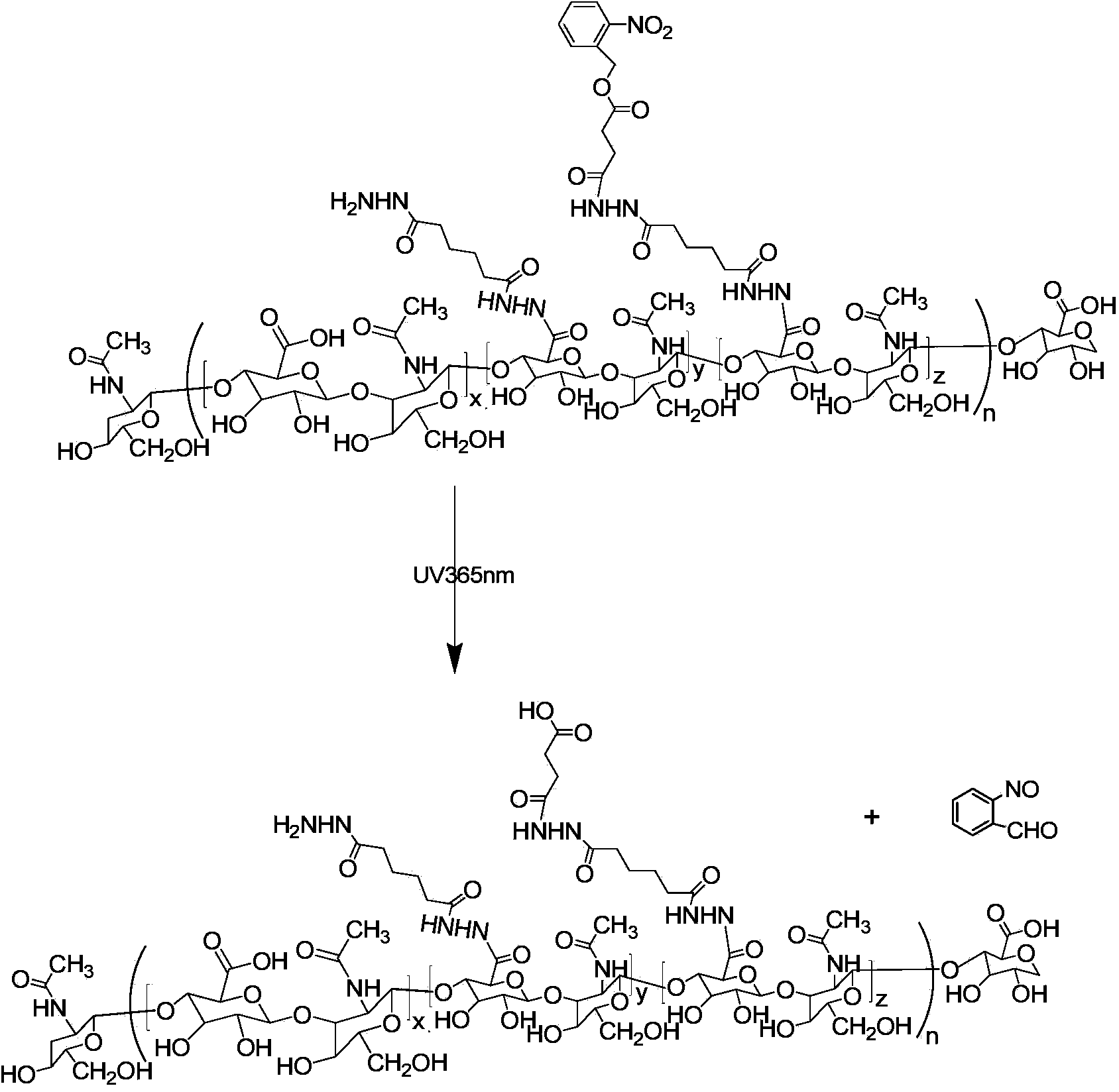
[0077] The present invention also provides a method for preparing a polymer having a structure of formula (I), comprising the following steps:
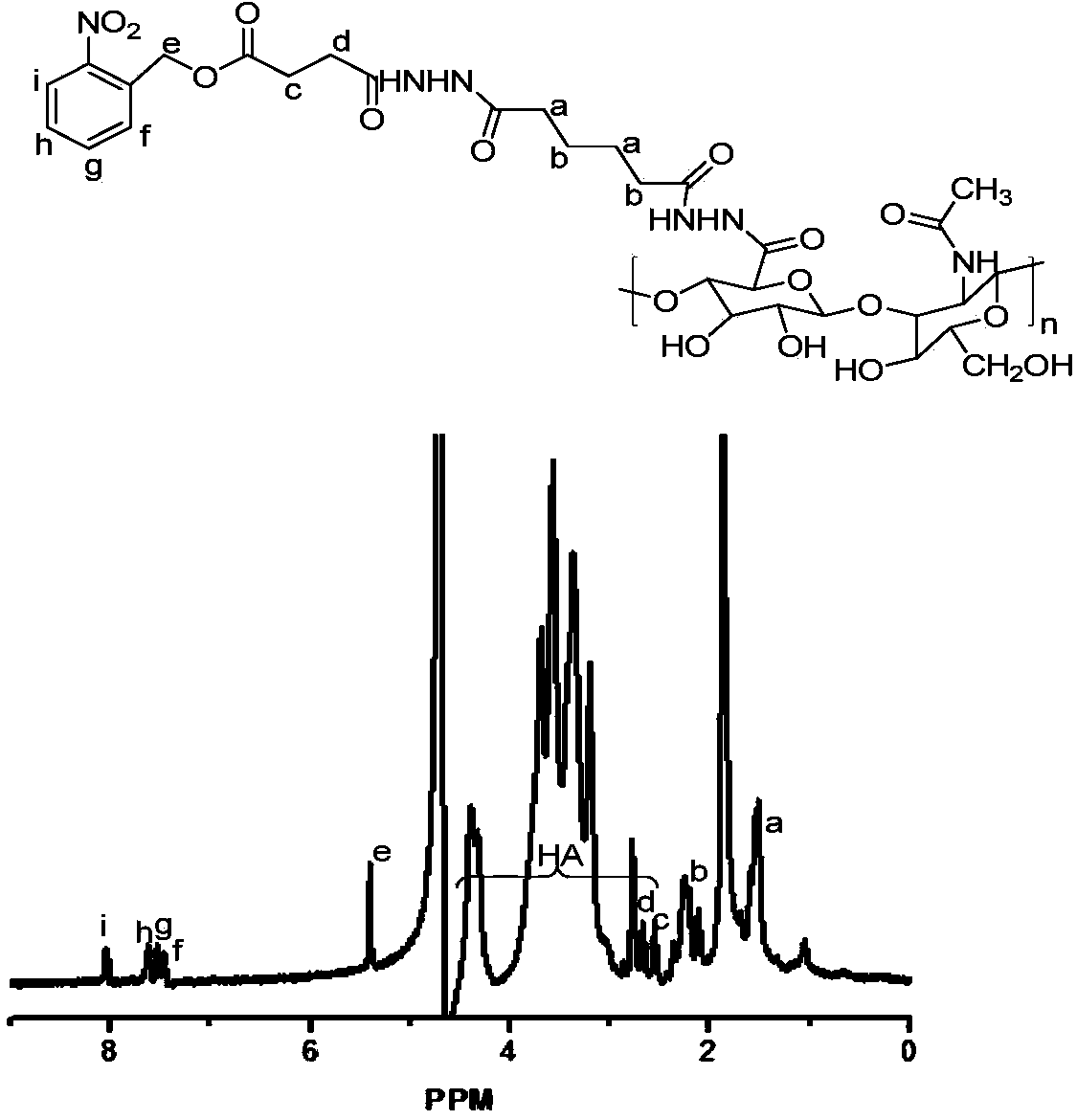
[0078] (1) Use adipic hydrazide (ADH) to chemically modify hyaluronic acid (HA) in an acidic aqueous solution to obtain a hyaluronic acid grafted adipic hydrazide polymer (HA-ADH) with a structure of formula (II) :
[0079]
[0080] Wherein, n is the degree of polymerization, and n is an integer between 25 and 500; x and y+z are relative mole numbers, and 0.5≤x / (x+y+z)<1.
[0081] The reaction formula is:
[0082]
[0083] Wherein, n is the degree of polymerization, and n is an integer between 25 and 500; x, y+z are relative moles, 0.5≤x / (x+y+z)<1;
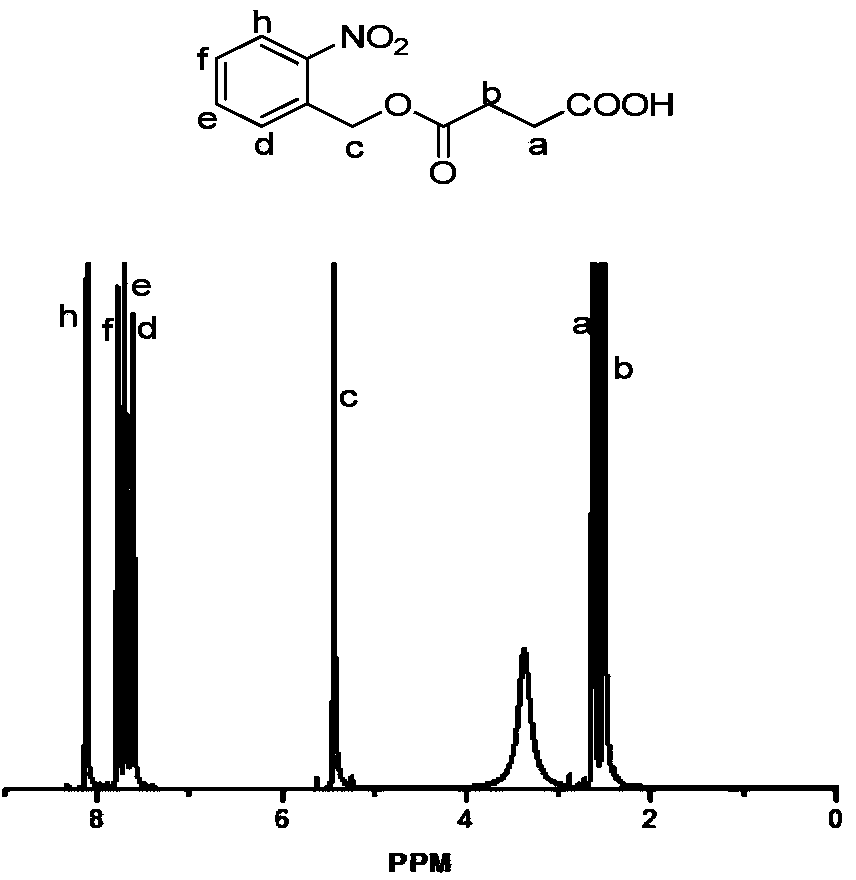
[0084] (2) Take o-nitrobenzyl alcohol and succinic anhydride to react at room temperature to obtain an o-nitrobenzyl alcohol derivative (NBS) with a structure of formula (Ⅲ), and then perform an activation reaction on the o-nitrobenzyl alcohol derivative to obtain O-nitrobenzyl a...
Embodiment 1
[0132] A photoresponsive micelle, the photoresponsive micelle includes an amphiphilic polymer and deionized water, the amphiphilic polymer uses hyaluronic acid as the hydrophilic main chain, and is grafted with o-nitrobenzyl alcohol derivatives The segment is the hydrophobic end. The synthesis of the photoresponsive micelles comprises the following steps:
[0133] (1) Dissolve 20g (0.05mol) of hyaluronic acid (HA, MW10K) in 5L of deionized water, add 44g (0.25mol) of adipic dihydrazide (ADH) to its aqueous solution, and use 0.1M hydrochloric acid solution under rapid stirring Adjust the pH to 4.5-4.8. Add 4.8g (0.025mol) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) in the above-mentioned reaction solution, then adjust the pH value at After 4.5-4.8, react at room temperature for 1 hour to obtain a white solid hyaluronic acid grafted adipic hydrazide polymer (HA-ADH). Take 10g HA-ADH and dissolve it in 2.4L formamide for later use;
[0134] (2) Dissolve ...
Embodiment 2
[0141] A photoresponsive drug-loaded micelle includes a photoresponsive micelle formed by an amphiphilic polymer and a hydrophobic anticancer drug. Among them, the mass ratio of the amphiphilic polymer to the hydrophobic anticancer drug is 1:0.1. The preparation method of the photoresponsive drug-loaded micelles is as follows:
[0142] Dissolve 10 mg of amphiphilic polymer with P1 structure in 10 ml of formamide, then dissolve 1 mg of chlorin e6 (Ce6) in 1 ml of formamide, and then add the formamide solution containing Ce6 dropwise to The amphiphilic polymer solution with the P1 structure was stirred and reacted for 30 minutes, and finally the above reaction solution was added to a dialysis bag with a molecular weight of 3500MW. After dialysis for 12 hours, the amphiphilic polymer-encapsulated chlorin e6 could be obtained. (Ce6) photoresponsive drug-loaded micelles.
[0143] The schematic diagram of the photoresponsive drug-loaded micelles is shown in Figure 6 As shown, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com