Method for preparing nitrogen-containing heterocyclic ring compound by use of chitin biomass
A technology of nitrogen heterocyclic compounds and chitin, which is applied in the field of preparation of high value-added nitrogen-containing heterocyclic compounds deoxyfructosazine, can solve the problems of poor selectivity of target products, complicated reaction steps, long reaction time, etc., and improve catalytic conversion efficiency , abundant reserves, and the effect of reducing the cost of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
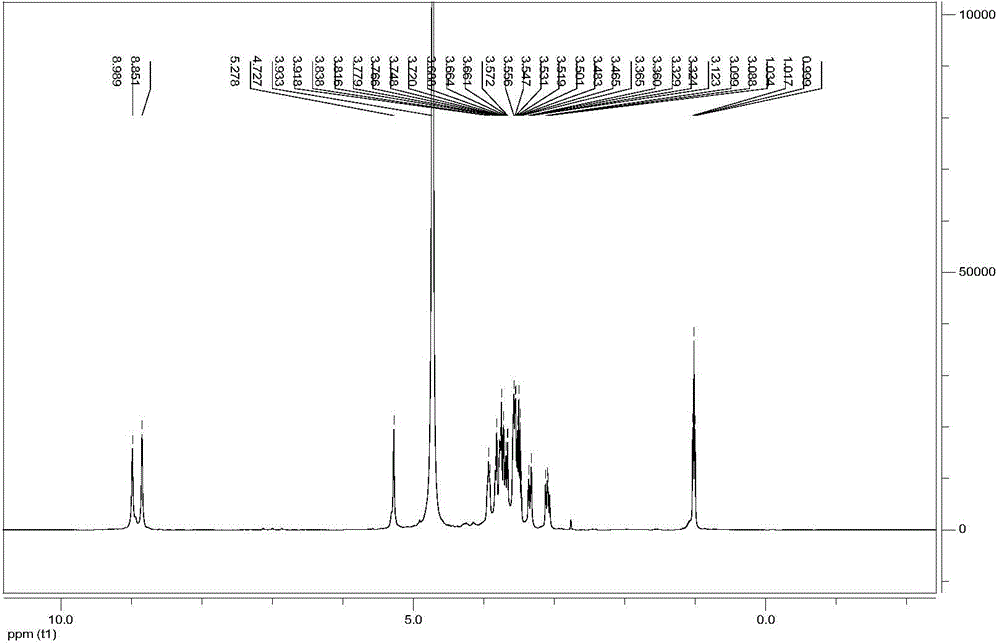
Image
Examples
Embodiment 1
[0022] (1) Prepare 30 g of an ethanol solution with a mass concentration of 12% 1-butyl-3-methylimidazolium hydroxide ionic liquid, add 10 g of D-glucosamine hydrochloride with a molecular weight of 215.5, add D-glucosamine Hydrochloride equimolar boric acid, after mixing evenly, react at 20°C for 8 hours.
[0023] (2) After the reaction, at room temperature, take 10 ml of the reaction product solution, add 50 ml of acetic anhydride and 30 ml of pyridine for acylation for 12 hours, then take 10 ml of the acylation reaction solution, extract three times, add 3×15 ml Ethyl acetate was used as the extractant to extract the acylated deoxyfructosine. The acylated product is obtained by rotary evaporation and concentration, the extractant is recovered, and the imidazole ionic liquid is recovered and reused;
[0024] (3) Add 1 g of the obtained acylated product to 5 ml of alkaline solution of 2 mol / L sodium hydroxide for deacetylation treatment. Under the condition of water bath, th...
Embodiment 2
[0026] (1) Prepare 45 g of an ethanol solution with a mass concentration of 50% 1-butyl-3-methylimidazolium hydroxide ionic liquid, add 35 g of D-glucosamine hydrochloride with a molecular weight of 215.5, and add D-glucosamine Hydrochloride and sodium hydroxide with an equal molar number were uniformly mixed and reacted at 80°C for 10 minutes.
[0027](2) After the reaction, at room temperature, take 2 ml of the reaction product solution, add 10 ml of acetic anhydride and 6 ml of pyridine for acylation for 15 hours, then take 10 ml of the acylation reaction solution, extract five times, add 5 ×20 ml tetrahydrofuran was used as the extractant to extract the acylated deoxyfructosazine.
[0028] (3) Concentrate the obtained ethyl acetate phase in which the product was dissolved, evaporate to dryness and concentrate, add 1 g of the obtained acylated product to 10 ml of sodium hydroxide alkaline solution with a concentration of 5 mol / L, and perform deacetylation treatment , under...
Embodiment 3
[0030] (1) Prepare 40 g of 1-butyl-3-methylimidazolium acetate ionic liquid with a mass concentration of 100%, add 35 g of chitin with a molecular weight of 250,000, and add glacial acetic acid whose molar mass is twice that of chitin , mixed evenly, and reacted at 150°C for 6 hours.
[0031] (2) After the reaction, at room temperature, take 3ml of the reaction product solution, add 15ml of acetic anhydride and 9ml of pyridine for acylation for 24 hours, then take 20ml of the acylation reaction solution, extract twice, add 2× 25 ml methyl isobutyl ketone was used as extractant to extract acylated deoxyfructosine.
[0032] (3) Concentrate the obtained methyl isobutyl ketone phase in which the product has been dissolved, evaporate to dryness and concentrate, add 1 g of the obtained acylated product to 15 ml of sodium hydroxide alkaline solution with a concentration of 10 mol / L, and carry out desorption Acetylation treatment, under water bath conditions, the reaction time is 30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com