Mycoplasma hyorhinosus strain and its inactivated vaccine and application
A technology of Mycoplasma hyorrhea and strains, applied in the directions of bacteria, antibacterial drugs, bacterial antigen components, etc., can solve the problems of increased mortality of weaned piglets, economic losses of pig farming, etc., and achieve good immunogenicity and stable titer. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
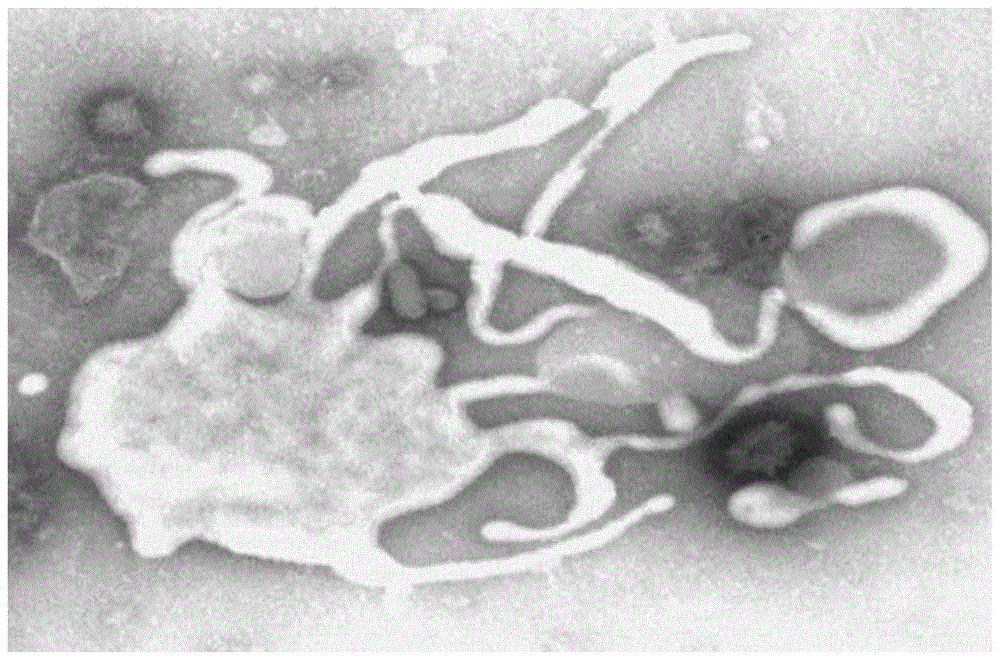
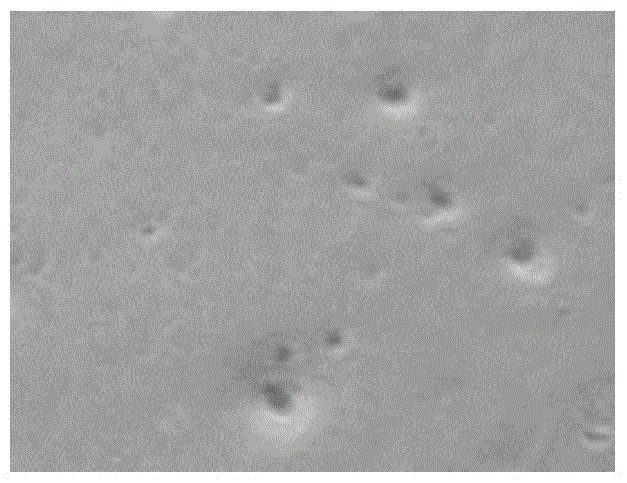
[0029] Example 1 Isolation and culture identification of Mycoplasma hyorhinois WH-C strain
[0030] 1 Sources and standards of strains
[0031] According to the declaration requirements of new biological products, combined with a large amount of test data obtained by the present invention, and referring to "Chinese Veterinary Pharmacopoeia" (2005 edition), the strains for seedling production were identified.
[0032] 1.1 Source of bacteria
[0033] The bacterial species used in the present invention is Mycoplasma hyorhinosum WH-C strain, which is obtained by the inventor after isolation and domestication from diseased pigs. Since this bacterial strain is produced from pregnant sows that have typical PMWS and suffer from respiratory syndrome Compared with other isolated strains, it has a higher proliferation titer on the medium and has better immunogenicity to pigs, so it is selected for vaccine production. The culture medium for Mycoplasma hyorhinosum (horse serum (HPS) is a...
Embodiment 2
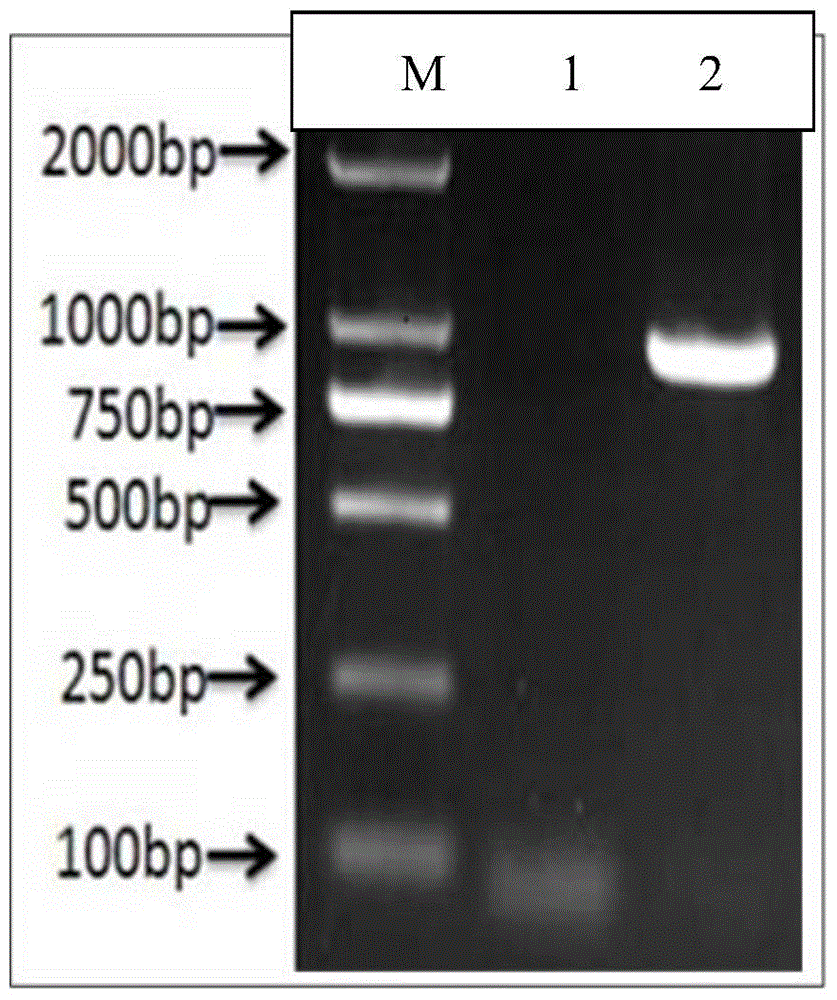
[0055] The preparation of embodiment 2 inactivated vaccine
[0056] The preparation process of the Mycoplasma hyorhini WH-C strain is prepared according to the current mature commercial vaccine preparation process. Judging from the quality inspection results of 3 batches of vaccines prepared in the inventor's laboratory and 5 batches of vaccines produced in the middle, the quality of the vaccine products prepared by this process flow is stable, and the efficacy inspection results fully meet the established quality standards.
[0057] 1 Preparation of bacteria solution
[0058] During the reproduction process, many factors will affect the proliferation of Mycoplasma hyorhinois WH-C strain. After a series of comparative experiments, the components of the culture medium for Mycoplasma hyorhinosum were selected as follows: horse serum (HPS) was a product of PAA Company, PPLO broth was a product of BD Company, and yeast extract was a product of British OXOID Company; of bacteria ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com