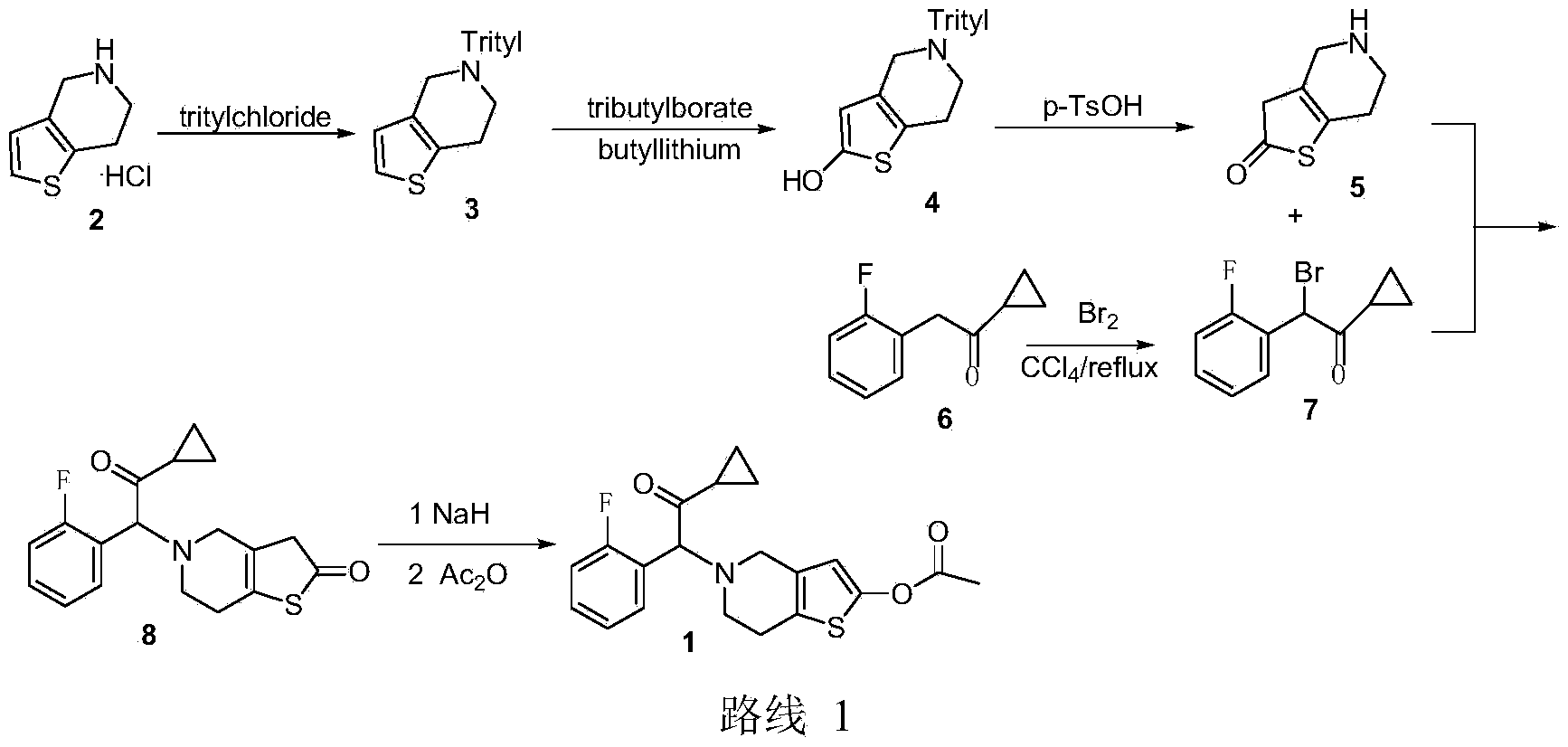
Synthetic method of prasugrel
A synthesis method and compound technology, applied in the field of synthesis of medicinal compounds, can solve the problems of slow reaction speed, low purity of condensation products, increased synthesis cost, etc., and achieve the effects of fewer by-products, fewer reaction steps and higher yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] 1-cyclopropyl-2-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl-2-(2-fluorobenzyl)ethanone hydrobromide
[0018]
[0019] Add 40 mL of acetonitrile and 6.76 g (38.9 mmol) of 4,5,6,7-tetrahydrothieno[3,2-c]-pyridine hydrochloride into a 100 mL two-necked flask, and add 20.0 g of potassium bicarbonate ( 0.2mol). After reacting for 30 minutes, a mixture of 10.03 g (39.0 mmol) of 2-bromo-1-cyclopropyl-(2-fluorophenyl)ethanone and 10 mL of acetonitrile was added dropwise, and the dropwise was completed in about 30 minutes. The reaction was carried out at room temperature for 25 hours, and the reaction was monitored by TLC. Filter off the solid, wash with 10mL of acetonitrile, then remove the solvent under reduced pressure at 50°C, add 20mL of acetone to the residue, cool to 10°C, add 6.5mL of 48% hydrobromic acid aqueous solution dropwise at this temperature, and dropwise add Precipitation was observed upon completion and stirring was continued for 3 hours at room temperature....
example 2
[0020] Example 21-Cyclopropyl-2-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl-2-(2-fluorobenzyl)ethanone hydrobromide
[0021] Add 40mL of acetonitrile and 6.76g (38.9mmol) of 4,5,6,7-tetrahydrothieno[3,2-c]-pyridine hydrochloride into a 100mL two-necked flask, and add 27.6g of potassium carbonate (0.2 mol). After reacting for 30 minutes, a mixture of 10.03 g (39.0 mmol) of 2-bromo-1-cyclopropyl-(2-fluorophenyl)ethanone and 10 mL of acetonitrile was added dropwise, and the dropwise was completed in about 30 minutes. The reaction was carried out at room temperature for 25 hours, and the reaction was monitored by TLC. Filter off the solid, wash with 10mL of acetonitrile, then remove the solvent under reduced pressure at 50°C, add 20mL of acetone to the residue, cool to 10°C, add 6.5mL of 48% hydrobromic acid aqueous solution dropwise at this temperature, and dropwise add Precipitation was observed upon completion and stirring was continued for 3 hours at room temperature. Cool to ...
example 35
[0022] Example 35-(α-cyclopropylcarbonyl-2-fluorobenzyl)-2-oxo-2,4,5,6,7,7a-hexahydrothieno[3,2-c]pyridine hydrobromide
[0023]
[0024] Add 15.70g (50.0mmol) 1-cyclopropyl-2-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl-2-( 2-fluorobenzyl)ethanone (20.0g compound with KHCO 3 solution free) and 60mL of anhydrous tetrahydrofuran, 34mL (2.2mol / L, 75mmol) n-butyllithium / n-hexane solution was added dropwise under stirring at 0°C, after stirring for 2 hours, cooled to -10°C, and 20.0mL ( 75mmol) a mixture of tributyl borate and 50mL tetrahydrofuran was reacted at -10°C for 1 hour, then further cooled to -20°C, 3.0mL (0.10mol) of 30% hydrogen peroxide was added dropwise, and the temperature was raised to room temperature and stirred for 12 hours. Extract with ethyl acetate (100mL×2), combine the organic layers, wash with water (100mL×3), dry over anhydrous sodium sulfate, spin dry the solvent under reduced pressure at 50°C, dissolve the mixture in 40mL acetone, cool to 10°C, at this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com