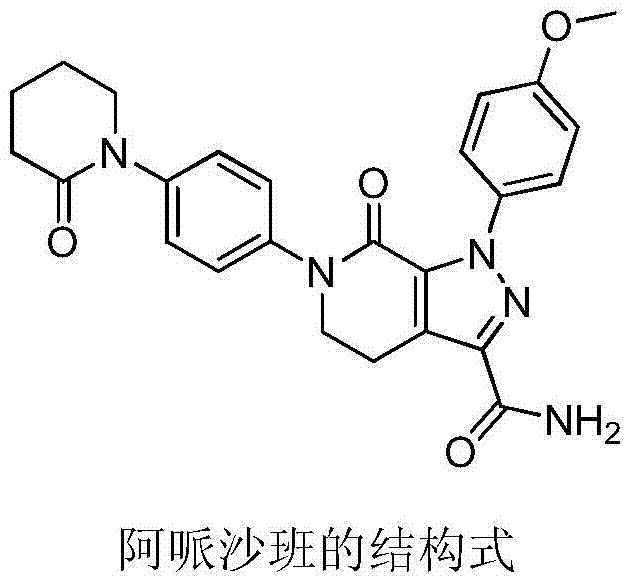
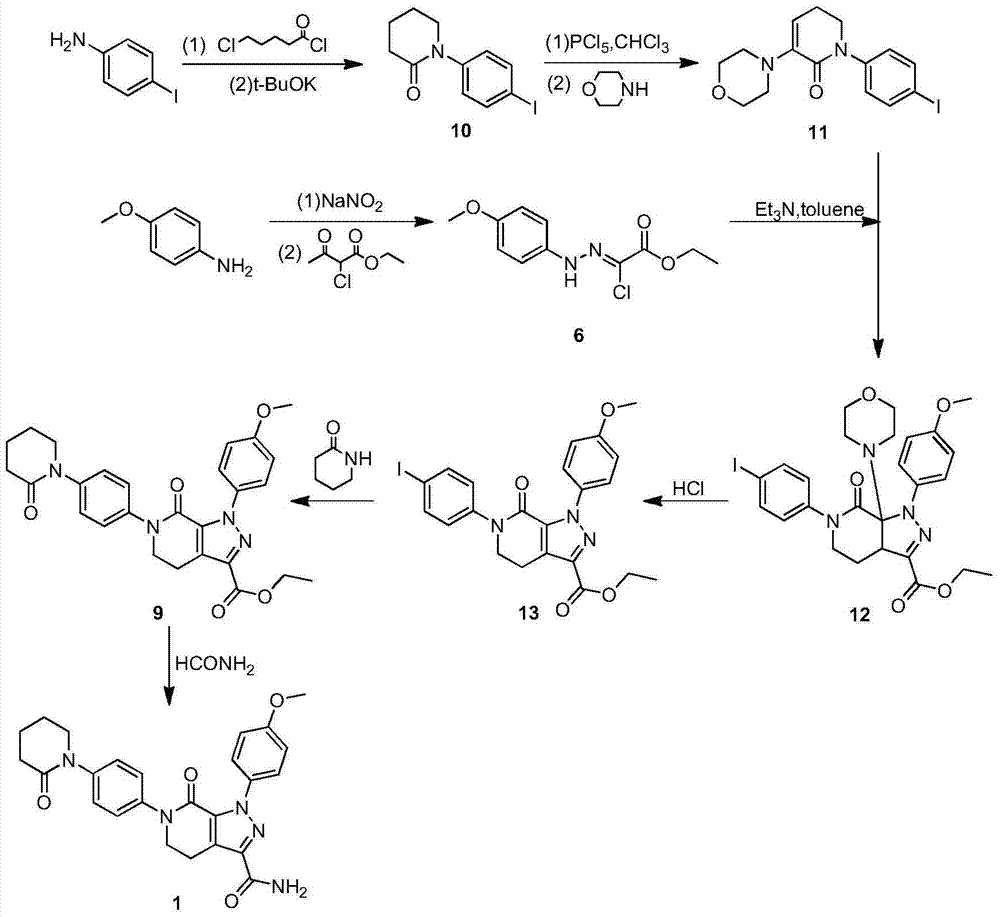
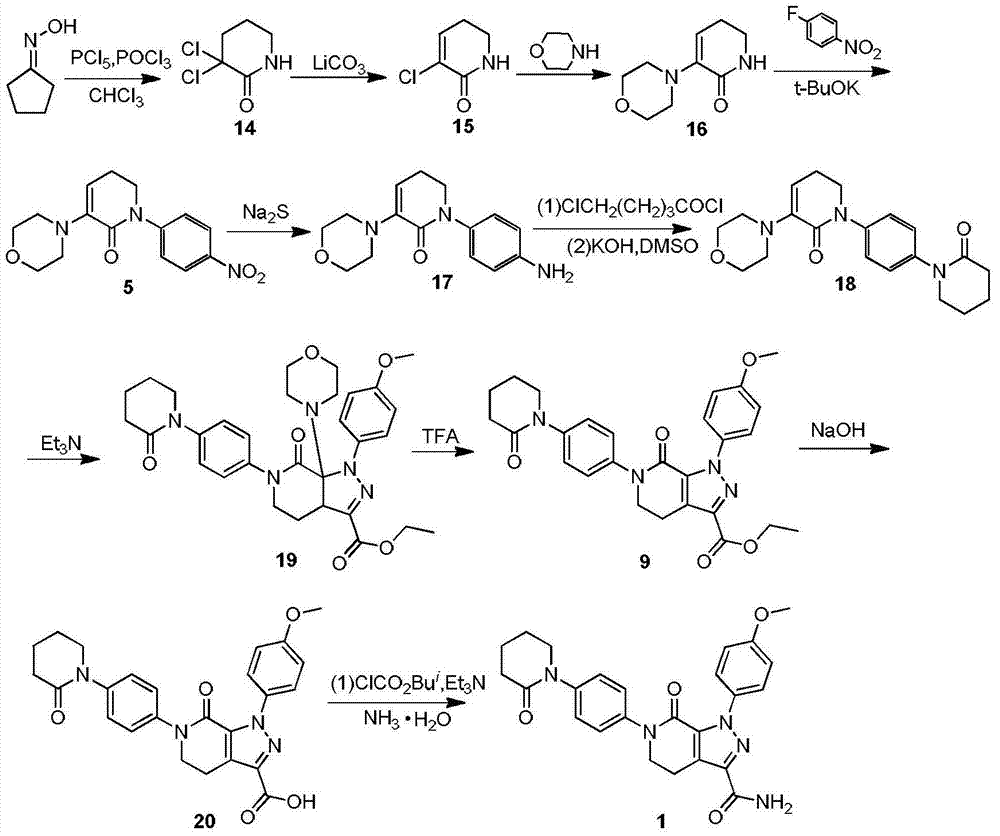
Preparation method of apixaban intermediate
A technology of apixaban and intermediates, applied in the direction of organic chemistry, etc., can solve the problems of long reaction time, complicated operation, and difficult industrialization, and achieve the effect of low price and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0059] The above content of the present invention will be further described in detail through the description of specific embodiments below. For those skilled in the art, it should not be understood that the scope of the above subject matter of the present invention is limited to the following method description; all technologies realized based on the above contents of the present invention belong to the scope of the present invention.
[0060] 1. Synthesis of 5-chloro-N-(4-nitrophenyl)pentanamide (2) (amidation reaction)
[0061]
[0062] To 360ml of anhydrous tetrahydrofuran, add 50g (0.36mol) of p-nitroaniline and 100mL (0.73mol) of triethylamine in sequence, and cool to 0°C in an ice-salt bath. Dissolve 67.3g (0.43mol) of 5-chloropentanoyl chloride in 100mL of anhydrous tetrahydrofuran, slowly add it dropwise to the reaction solution, and react at room temperature for 4 hours. Most of the solvent was evaporated, poured into 500mL ice water under stirring, a large amoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com