Method of preparing furfural oxime acid
A technology of furoxamic acid and furylacetic acid, applied in the direction of organic chemistry, can solve the problems of low yield, difficult to realize reaction conditions, unsuitable for industrial production, etc., achieve high yield, reduce reaction steps, and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
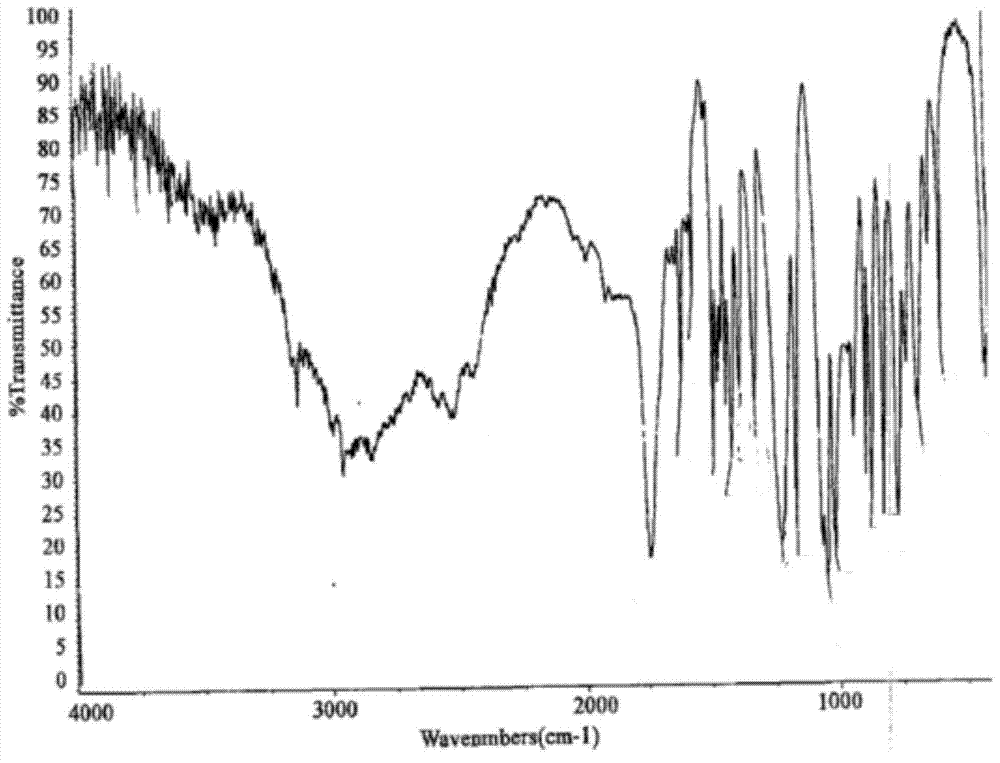
Image
Examples
Embodiment 1
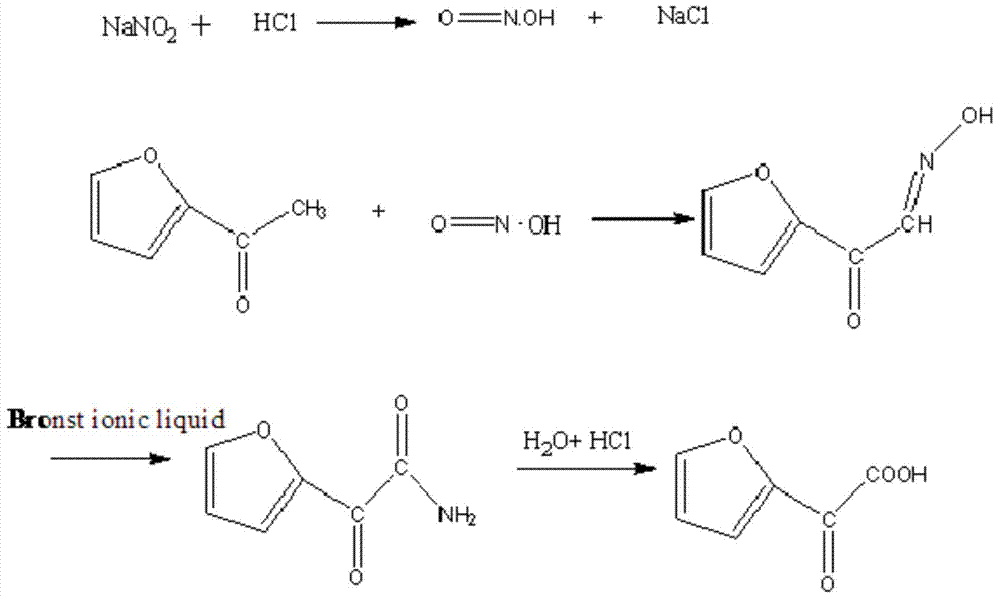
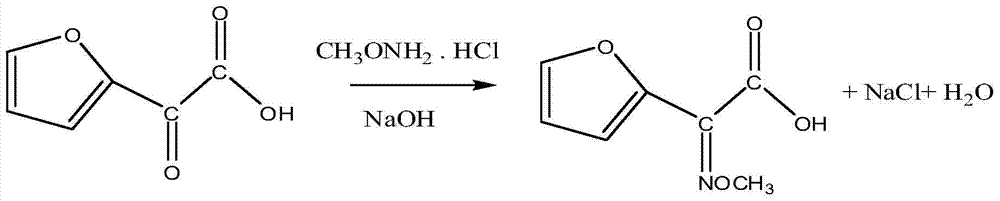
[0042] (1) Preparation of 2-oxo-2-furylacetic acid:
[0043] Add 110g (1.0mol, M=110g / mol) 2-acetylfuran, 400mL water, and 93.5g sodium nitrite (1.5mol, M=69g / mol) into a three-necked flask equipped with a stirrer, condenser, and thermometer, and stir Dissolve and heat up to 30°C, start to add 150g of 36% hydrochloric acid (M=37.5g / mol) dropwise, with the addition of hydrochloric acid, the reaction temperature gradually rises, cool with cooling water to control the reaction temperature at 4°C, and add hydrochloric acid evenly for 3 hours In the reaction flask, the dropwise addition was completed and the heat preservation reaction was carried out for 2 hours. At this time, the pH value of the reaction solution was about 4.0. Add 4.4 g of ionic liquid 1,3-dimethylimidazolium bisulfate catalyst and react for 1 hour. Add dichloromethane and stir Extract for 0.5h, let stand to separate layers. The organic phase is distilled to recover dichloromethane, and the unreacted 2-acetylfur...
Embodiment 2
[0048] (1) Preparation of 2-oxo-2-furylacetic acid:
[0049] Add 110g (1.0mol, M=110g / mol) 2-acetylfuran, 400mL water, and 93.5g sodium nitrite (1.5mol, M=69g / mol) into a three-necked flask equipped with a stirrer, condenser, and thermometer, and stir Dissolve and heat up to 30°C, start to add 120g of 36% hydrochloric acid (1.15mol, M=37.5g / mol) dropwise, with the addition of hydrochloric acid, the reaction temperature gradually rises, the cooling water is cooled to control the reaction temperature at 4°C, and control it for 3 hours Add hydrochloric acid evenly in the reaction bottle, and keep the temperature for 2 hours after the dropwise addition. At this time, the pH value of the reaction solution is about 4.0. Add 5.5 g of ionic liquid 1-butyl-3-methylimidazole trifluoromethanesulfonate catalyst, and react 1 Hours, dichloromethane was added, stirred and extracted for 0.5h, and the layers were left to stand. The organic phase is distilled to recover dichloromethane, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com