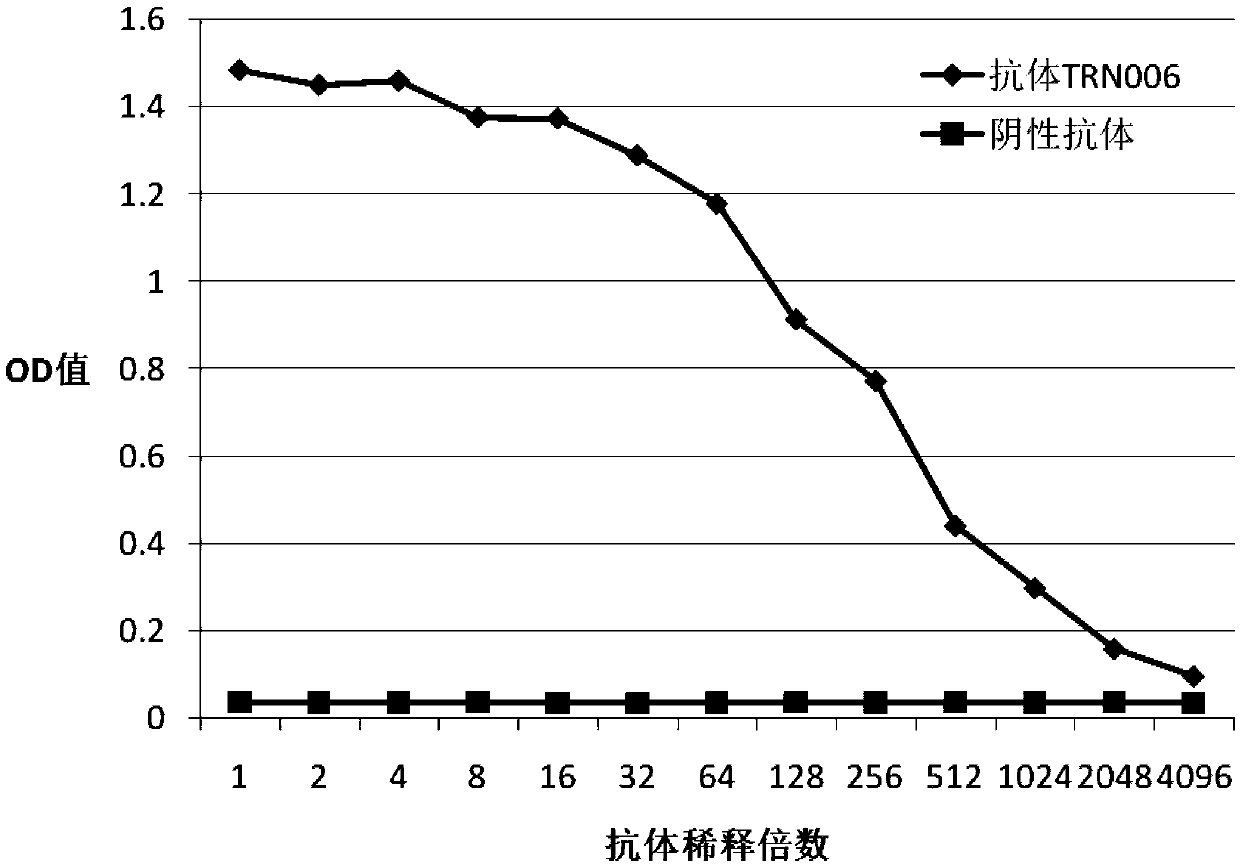
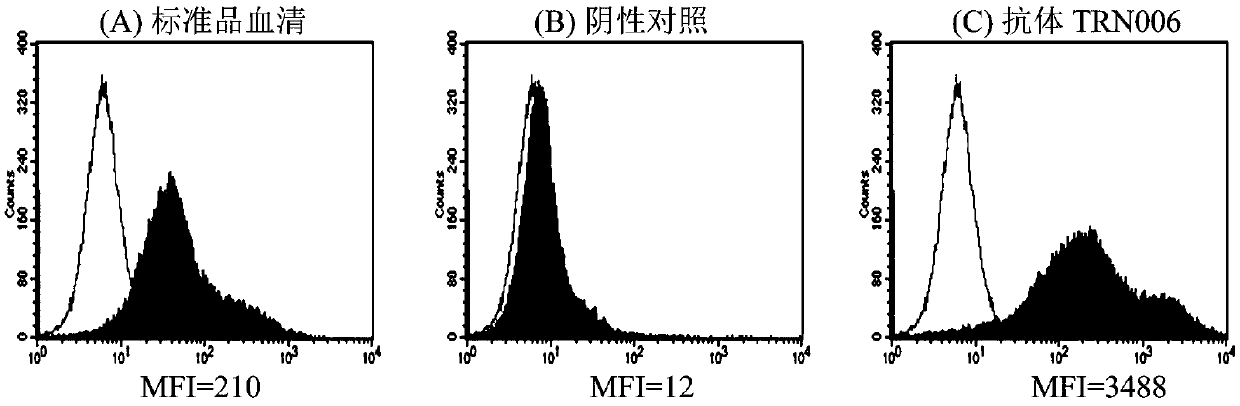
A fully human neutralizing antibody against rabies virus
A rabies virus, fully human technology, applied in the fields of cellular immunology and molecular biology, can solve the problems of long production cycle, low antibody affinity and limited success rate, and achieve good neutralization effect, good specificity and affinity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The preparation process of the fully human monoclonal anti-rabies virus neutralizing antibody TRN006 described in the embodiments of the present invention comprises the following steps:
[0019] 1. Lymphocyte Isolation and Single Memory B Cell Sorting
[0020] Collect 100ml of venous blood from two volunteers who have been injected with rabies vaccine (Novartis batch number: 1928) and have produced protective antibodies, put them in anticoagulant tubes containing heparin, and use density centrifugation to separate mononuclear cells (PBMCs) ), after cell counting, single B cells of CD3-, CD14-, CD16-, CD19+, CD27+ were sorted from PBMC by BDFACSria flow cytometer (BD Biosciences, San Jose, CA) into a 96-well PCR plate containing RT reaction system, Make each well contain one memory B cell, and freeze the PCR plate at -80°C for future use.
[0021] 2. Isolation of Antibody Variable Region Genes from Single B Cells by RT-PCR
[0022] 1) Synthesize the first strand of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com