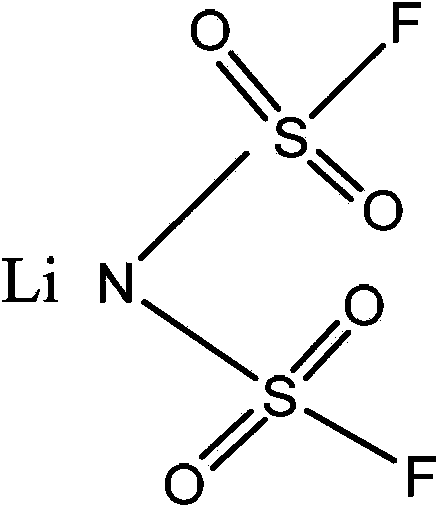
Method for preparing bis(fluorosulfonyl)imide
A technology of lithium bisfluorosulfonimide and lithium bisfluorosulfonimide is applied in the field of preparation of lithium bisfluorosulfonimide, which can solve the problems of difficult purification, difficult separation, and potential safety hazards of products, and achieves The effect of easy industrial preparation and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under the protection of dry nitrogen, add 219g (1mol) of bisfluorosulfonimide potassium salt, 194g (1mol) of lithium bisoxalate borate and 1000ml dimethyl carbonate into the three-necked flask, stir and react at room temperature for 12 hours, and filter under reduced pressure to remove insoluble After the filtrate was concentrated to about 150ml under reduced pressure, an equal volume of dichloromethane was added, and after cooling down to room temperature, 150g of lithium bisfluorosulfonimide was obtained after filtration, washing and drying.
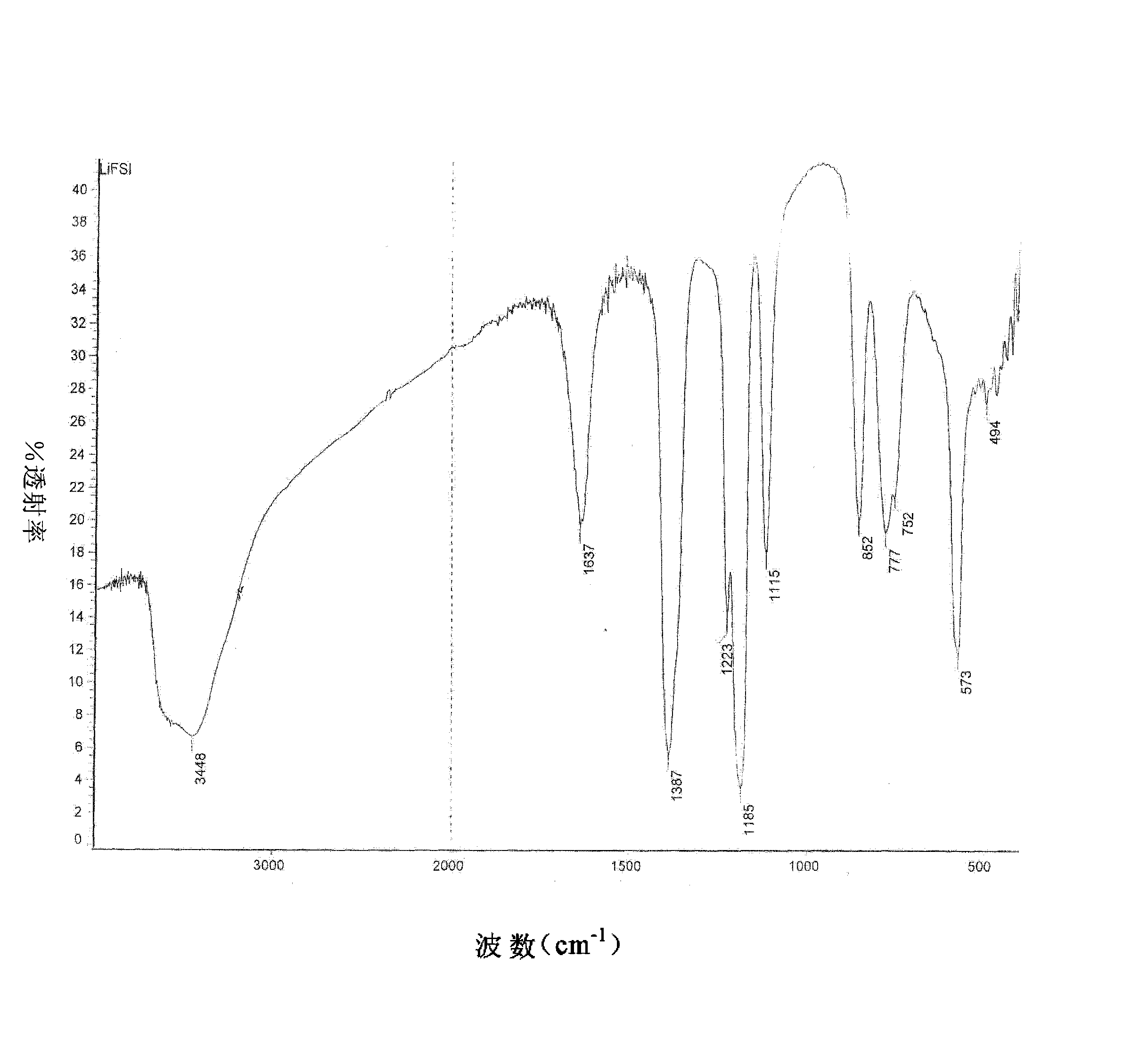
[0032] The product has been tested by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 , 573cm -1 By comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of lithium bisfluorosulfonimide.
Embodiment 2
[0034] Under the protection of dry nitrogen, add 219g (1mol) of bisfluorosulfonimide potassium salt, 144g (1mol) of lithium oxalate difluoroborate and 1000ml dimethyl carbonate into the three-necked flask, stir and react at room temperature for 12 hours, and remove by filtration under reduced pressure For insoluble matter, the filtrate was concentrated to about 150ml under reduced pressure, then an equal volume of dichloromethane was added, and after cooling down to room temperature, 138g of lithium bisfluorosulfonyl imide was obtained after filtration, washing and drying.
[0035] The product has been tested by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 ,573cm -1 By comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of lithium bisfluorosulfonimide.
Embodiment 3
[0037] Under the protection of dry nitrogen, add 219g (1mol) of bisfluorosulfonimide potassium salt, 194g (1mol) of lithium bisoxalate borate and 1200ml of ethyl methyl carbonate to the three-necked flask, stir and react at room temperature for 12 hours, and filter under reduced pressure to remove insoluble After the filtrate was concentrated to about 165ml under reduced pressure, an equal volume of dichloromethane was added, and after cooling down to room temperature, 158g of lithium bisfluorosulfonimide was obtained after filtration, washing and drying.
[0038] The product has been tested by infrared, at 1387cm -1 ,1223cm -1 ,1185cm -1 ,852cm -1 ,777cm -1 , 573cm -1 By comparing the standard spectrum of bisfluorosulfonimide, it was determined to be the characteristic group of lithium bisfluorosulfonimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com