Non-steroid antiandrogen compounds as well as preparation method and application thereof
An anti-androgen, compound technology, applied in the field of medicine, can solve the problem of expensive treatment, and achieve the effect of inhibiting tumor growth, large clinical application value, and high social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
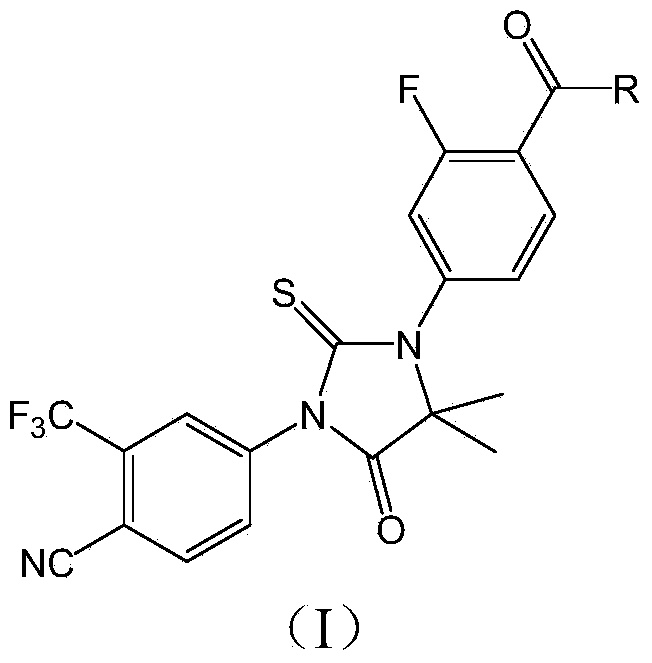
[0038] Example 1: The new compound 5,5-dimethyl-1-(2-fluoro-3-hexanoyl)phenyl-3-(2-trifluoromethyl-3-cyano)phenyl-4-oxo -Synthesis of 2-thioimidazolidinone
[0039]
[0040] 1. Synthesis of Intermediate 2
[0041] At 0°C, the reactant 3-fluoro-4-iodoaniline (3-flouro-4-iodoaniline) (474mg, 2.0mmol) was added to a solution of acetone (116mg, 2.0mmol), AcOH (3.2ml) , keep the reaction at 0°C in the dark for 15-30min, add trimethylsilyl nitrile (TMSCN) (0.26ml, 2.0mmol) in the dark, react overnight at room temperature, add the reaction solution into ammonia water (ice bath) pH is alkaline , extracted three times with dichloromethane, combined the organic phases, washed the organic phases with water and brine, and anhydrous Na 2 SO 4 Dry, and remove solvent by rotary evaporation to obtain Intermediate 2;
[0042] 2. Synthesis of Intermediate 4
[0043] Intermediate 2 (617.9mg, 2.03mmol) was added to a microwave reaction vial, dried and freshly evaporated DMF (1ml), and the...
Embodiment 2
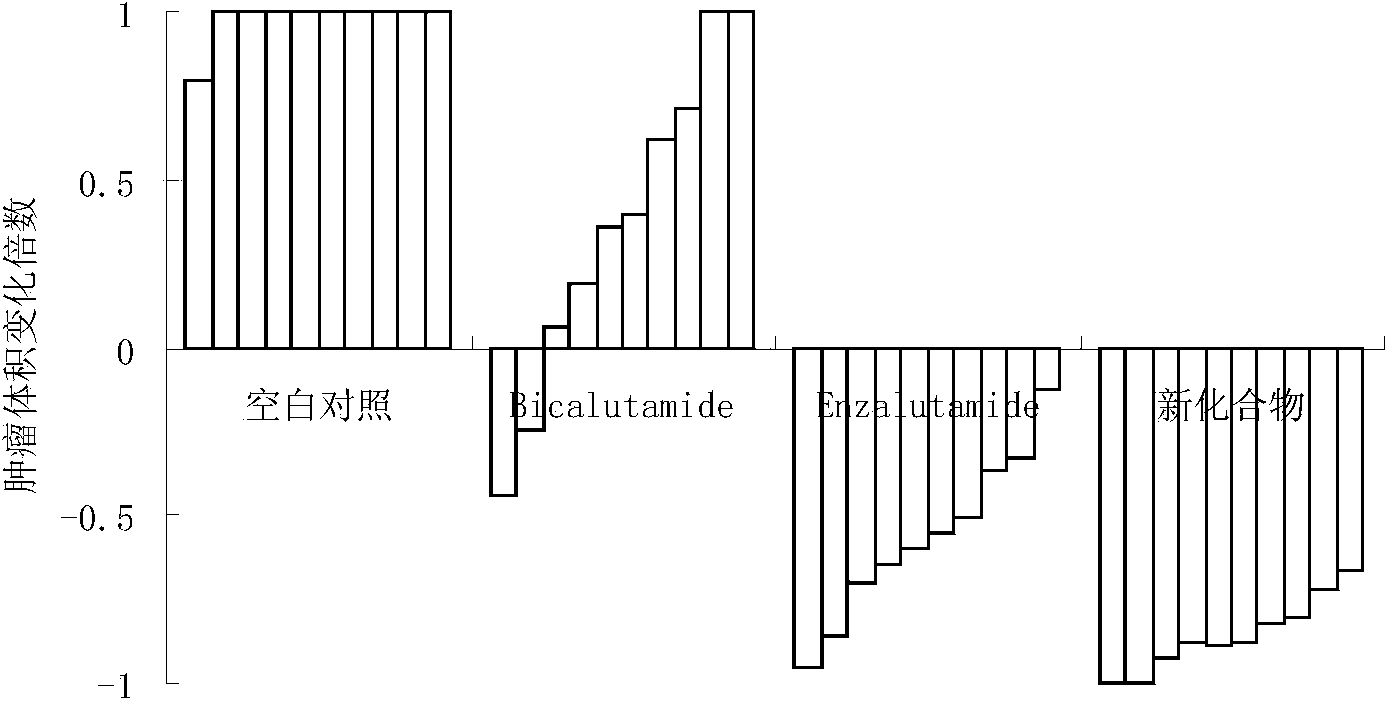
[0052] Embodiment 2: the pharmacological experiment of compound of the present invention:
[0053] 1. In vitro experiments to study the inhibitory effect of new drugs on prostate cancer epithelial cells
[0054] 1. Materials and reagents
[0055] Androgen-independent prostate cancer (LNCaP-AI) cells were donated by the Urology Department of Changhai Hospital Affiliated to the Second Military Medical University of the Chinese People's Liberation Army.
[0056] RPMI1640 culture medium (Hyclone company),
[0057] Activated carbon-treated fetal bovine serum, Charcoal Stripped Serum, CSS (Hyclone Company),
[0058] Elecsys total PSA reagent Kit (Roche Company, Cat.No.1731262),
[0059] Enzalutamide and Bicalutamide were purchased from Shanghai Hanxiang Biotechnology Co., Ltd.,
[0060] The new compound is 5,5-dimethyl-1-(2-fluoro-3-hexanoyl)phenyl-3-(2-trifluoromethyl-3-cyano)phenyl- 4-Oxo-2-thioimidazolinone (compound three).
[0061] 2. Experimental method
[0062] (1), MT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com