Thienyl phosphorescent iridium complex as well as preparation method and application thereof
A technology of thiophene-based phosphorescence and iridium complexes, applied in the field of material science, can solve the problems of light instability and easy decomposition, and achieve the effects of good stability and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
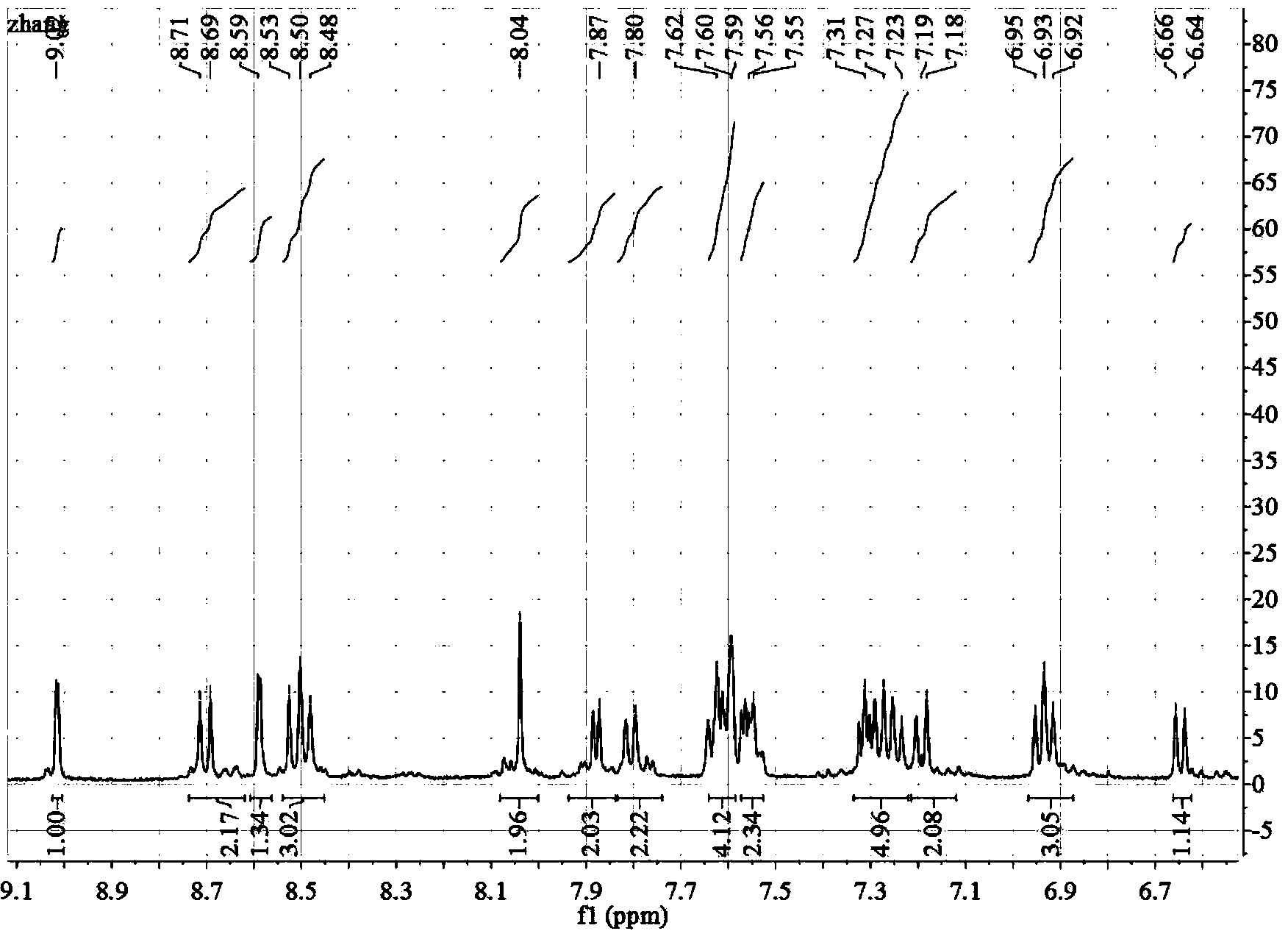
[0027] Preparation of iridium complex 3,8-(2-thiophene)-phenanthroline iridium-2-phenylpyridine complex: Weigh 1 mmol of 3,8-dibromophenanthroline iridium-2-phenylpyridine complex And 2.4mmol of 2-thiophene pinacol borate; Add the tetrakis (triphenylphosphine) palladium that molar number is 5%; Add the sodium carbonate aqueous solution 20mL that concentration is 2M wherein; Add the DMF of 40mL, temperature 70 ℃, Stir for 36 hours; after the reaction, cool the reaction solution to room temperature, pour it into water, and stir for 30 minutes; use dichloromethane to extract the water phase several times, combine the organic phase, and dry the organic phase with anhydrous magnesium sulfate; filter the above solution , the filtrate was removed, and the solvent was removed in vacuo. The crude product was obtained, and the pure product was obtained by thin-layer column chromatography.
[0028] The H NMR spectrum of the complex is characterized by figure 1 shown.
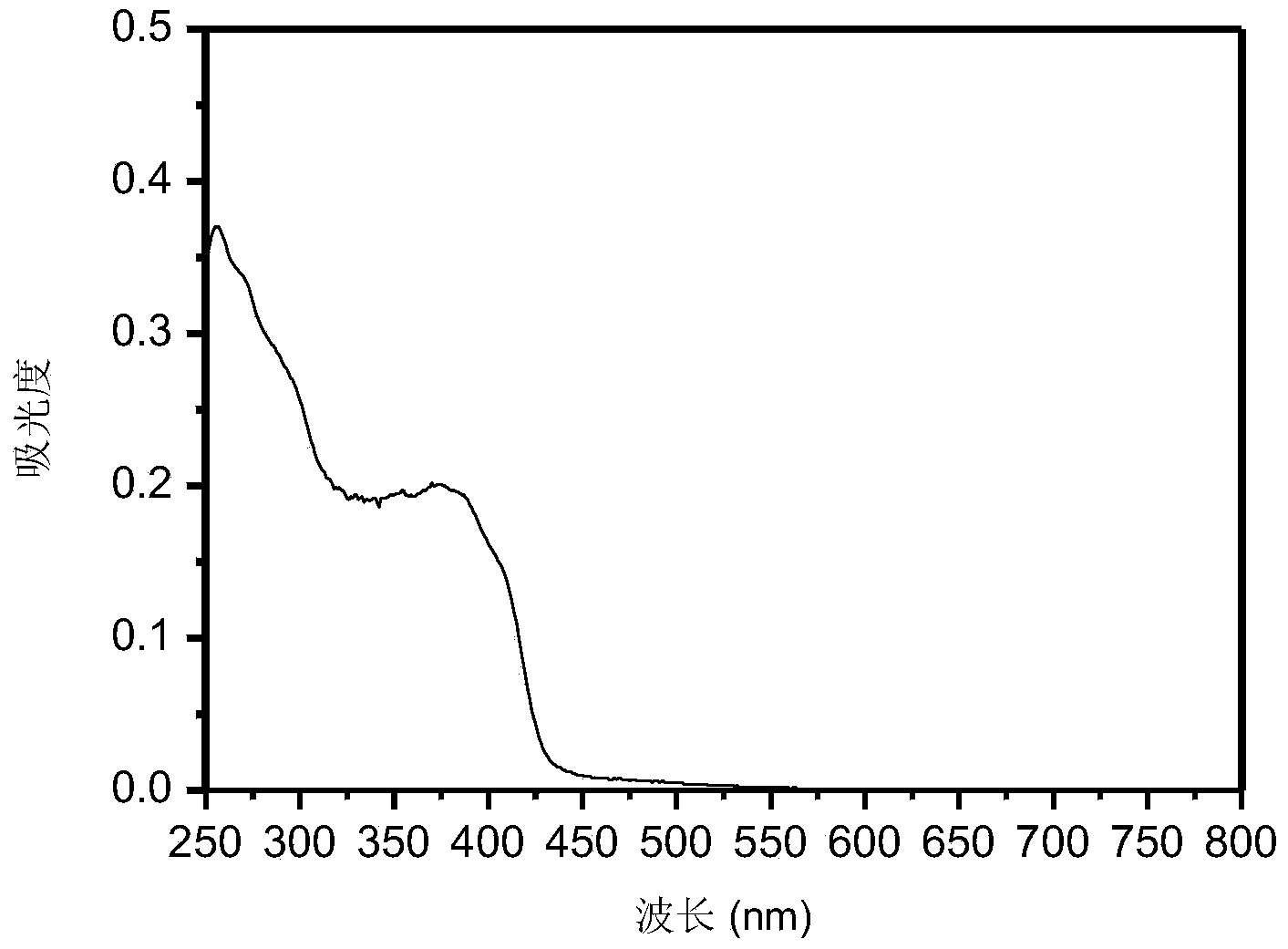
[0029] Its abso...
Embodiment 2
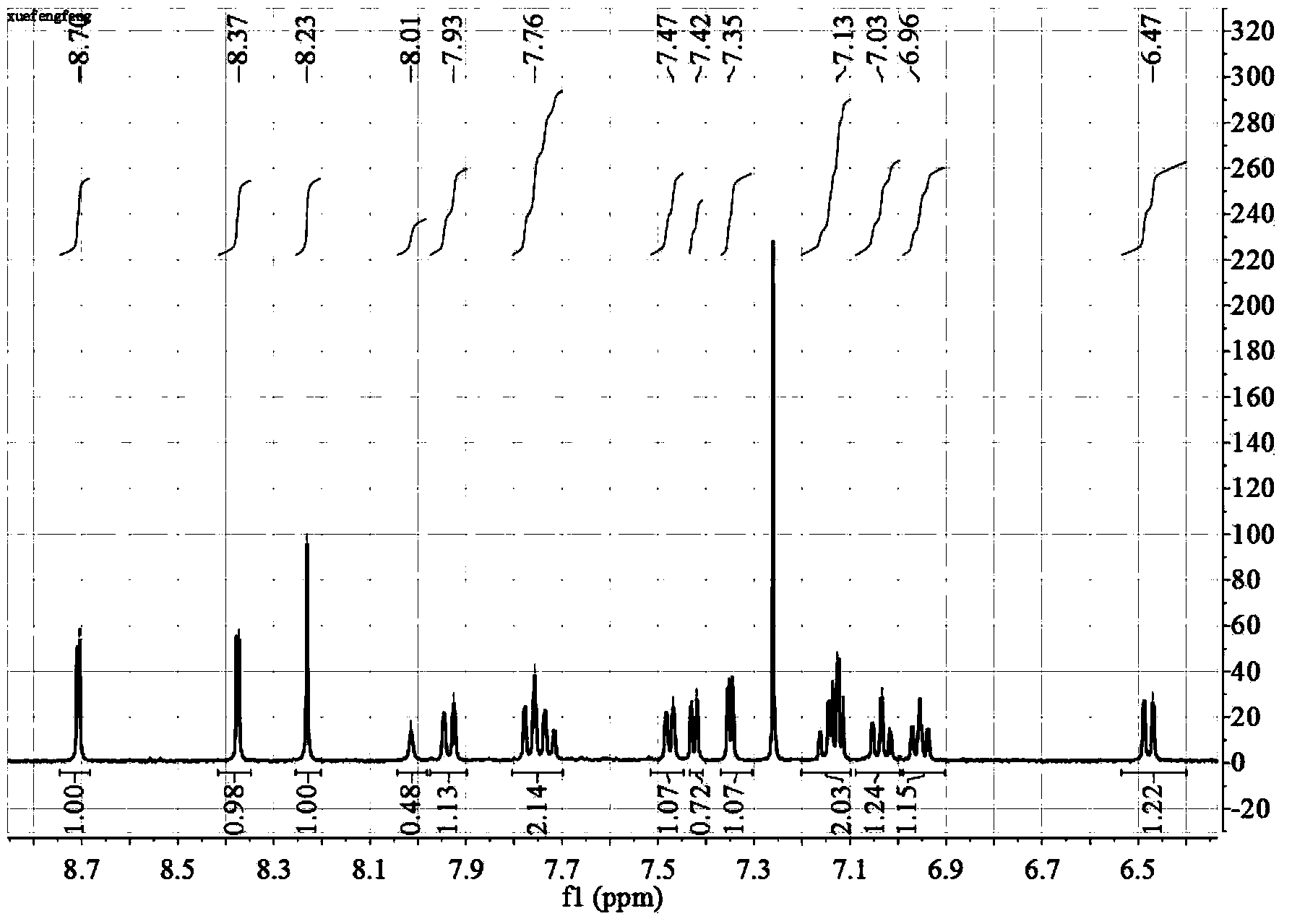
[0031] Preparation of iridium complex 3,8-(2-thiophene)-o-phenanthroline iridium-2-phenylquinoline complex: Weigh 3,8-dibromophenanthroline iridium-2-phenylpyridine complex 1mmol and 2.4mmol of 2-thiophene pinacol borate; add tetrakis(triphenylphosphine) palladium with 5% molarity; add 20mL of aqueous sodium carbonate solution with a concentration of 2M; add 40mL of DMF at a temperature of 70°C , stirred for 36 hours; after the reaction, the reaction solution was cooled to room temperature, poured into water, and stirred for 30 min; with dichloromethane, extracted the water phase several times, combined the organic phase, and dried the organic phase with anhydrous magnesium sulfate; filtered the above solution, the filtrate was removed, and the solvent was removed in vacuo. The crude product was obtained, and the pure product was obtained by thin-layer column chromatography.
[0032] The H NMR spectrum of the complex is characterized by figure 2 shown.
[0033] Its absorpt...
Embodiment 3
[0034] Example 3: Singlet oxygen generation experiment
[0035] The singlet oxygen generation experiment was carried out in a mixed solution of ethanol: water = 4:1 volume ratio, and the singlet oxygen produced by the complex under near-infrared light and 1,3-diphenylisobenzofuran (DPBF) were investigated. Reaction, the production of singlet oxygen is indicated by consumption of 1,3-diphenylisobenzofuran, and the change of ultraviolet absorption at 410nm indicates the ability of singlet oxygen generation. The results are as follows Figure 7 and Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com