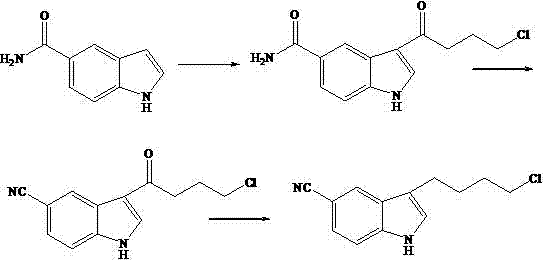
New synthetic process of vilazodone intermediate 5-cyano-3(4-chlorobutyl)-indole
A technology of vilazodone and chlorobutyl is applied in the field of new synthesis process of vilazodone intermediate 5-cyano-3(4-chlorobutyl)-indole, and achieves great economic and social benefits, equipment Simple, environmentally friendly production procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 2 Synthesis of 5-cyano-3 (4-chloro-butyryl)-indole
[0019] Add 400 L of solvent dichloromethane and 60.0 kg of raw material 5-amino-formyl-3(4-chloro-butyryl)-indole into the enamel reaction kettle, and add phosphorus oxychloride in an ice-salt bath to control the temperature below 10 °C 30.0 kg, added in about 0.5 h. Slowly raise the temperature and reflux the reaction for 4-6 h, and the intermediate 5-amino-formyl-3(4-chloro-butyryl)-indole spot disappears as monitored by thin-layer chromatography, and the dehydration reaction is considered complete. Then lower the temperature below 10°C, and add 5% sodium hydroxide aqueous solution to adjust the pH value to about 8. Stand to separate the layers, separate the dichloromethane, then extract once with 200 L of dichloromethane, combine the organic phases, dry, and concentrate under reduced pressure to obtain 5-cyano-3(4-chloro-butyryl)-indole Intermediate crude product 51.0 kg.
Embodiment 2
[0020] Example 3 Synthesis of 5-cyano-3 (4-chlorobutyl)-indole
[0021] Add 400 L of solvent dichloromethane and 50.0 kg of raw material 5-cyano-3(4-chloro-butyryl)-indole into the enamel reaction kettle. After stirring and dissolving, add 5.1 kg of diborane at room temperature for about 1 h, then slowly raised to reflux for 3 h, the spot of the intermediate 5-cyano-3 (4-chloro-butyryl-indole) disappeared as monitored by thin-layer chromatography, and the reduction reaction was considered complete. Concentration under reduced pressure gave 45.6 kg of solid 5-cyano-3 (4-chlorobutyl-indole) crude product. The crude product was dissolved in 200 L of methanol, filtered, concentrated, cooled to crystallize, filtered, and dried to obtain 41.2 kg of 5-cyano-3(4-chlorobutyl)-indole fine product.
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com