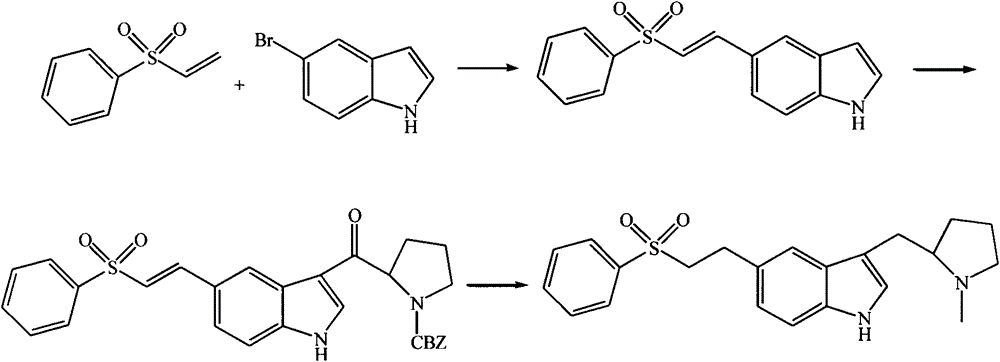
Synthetic process of eletriptan
A technology for eletriptan and a synthesis method is applied in the synthesis field of 5-hydroxytryptophan receptor inhibitor eletriptan, and achieves the effects of green environmental protection production procedure, great economic and social benefits, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15-
[0016] The synthesis of embodiment 15-[2-(benzenesulfonyl) vinyl]-1H-indole
[0017] In a 300L enamel reaction kettle, add 150L of N,N-dimethylformamide, 20kg of benzenesulfonylethylene and 25kg of 5-bromoindole as the reaction raw materials, and the catalyst PdCO 3 Add 0.5kg at one time, 6kg of catalyst triphenylphosphine, 25kg of acid-binding agent triethylamine, stir and heat to 100°C for 6h, and monitor the complete reaction of 5-bromoindole by TLC. The reaction system was naturally lowered to room temperature, and about 100L of dichloromethane was added to dilute and filtered. The filtrate was extracted twice with 50L of brine, and the organic phase was dried overnight with anhydrous sodium sulfate. The organic phase was collected and concentrated to dryness under reduced pressure. The solid product was then crystallized with a mixed solvent of acetone-petroleum ether=1:1 to obtain the product intermediate 5-[2-(benzenesulfonyl)vinyl]-1H-indole.
Embodiment 2
[0018] Example 2 Synthesis of intermediate 3-(N-CBZ-2-pyrrolidinyl)formyl-5-(2-benzenesulfonyl-vinyl-1-yl)-1H-indole
[0019] Add 25kg of the intermediate 5-[2-(benzenesulfonyl)vinyl]-1H-indole to a 300L enamel reaction kettle, dissolve 100L in dried dichloromethane, then control the temperature within 10°C, and add N- The complex synthesized by CBZ-D-prolyl chloride and anhydrous aluminum trichloride in methylene chloride at a low temperature below 5°C is 50L, in which N-CBZ-D-prolyl chloride and anhydrous aluminum trichloride The ratio is 1:1.5, containing 20kg of N-CBZ-D-prolyl chloride. After adding the complex, continue to react at room temperature for 5h, and the intermediate raw material point disappears as monitored by TLC, indicating that the reaction is complete. Control the temperature at 10°C, first add 50L of ice water to hydrolyze, wash and extract once, then adjust the pH to about 7 with saturated sodium carbonate solution, extract once, and finally extract with...
Embodiment 3
[0020] The synthesis of embodiment 3 eletriptan
[0021] Add intermediate 3-(N-CBZ-2-pyrrolidinyl)formyl-5-(2-benzenesulfonyl-vinyl-1-yl)-1H-indole 25kg in 300L enamel reaction kettle, 75L of solvent was dissolved, and 15kg of reducing agent SnCl was added 2 and catalyst 25% ammonium chloride aqueous solution 75L, reflux reaction for 4h, thin-layer chromatography monitors that the intermediate raw material point disappears, and the reaction is completed. Adjust the pH value to about 8 with saturated sodium carbonate solution, add 50 L of dichloromethane for extraction twice, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain crude eletriptan.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com