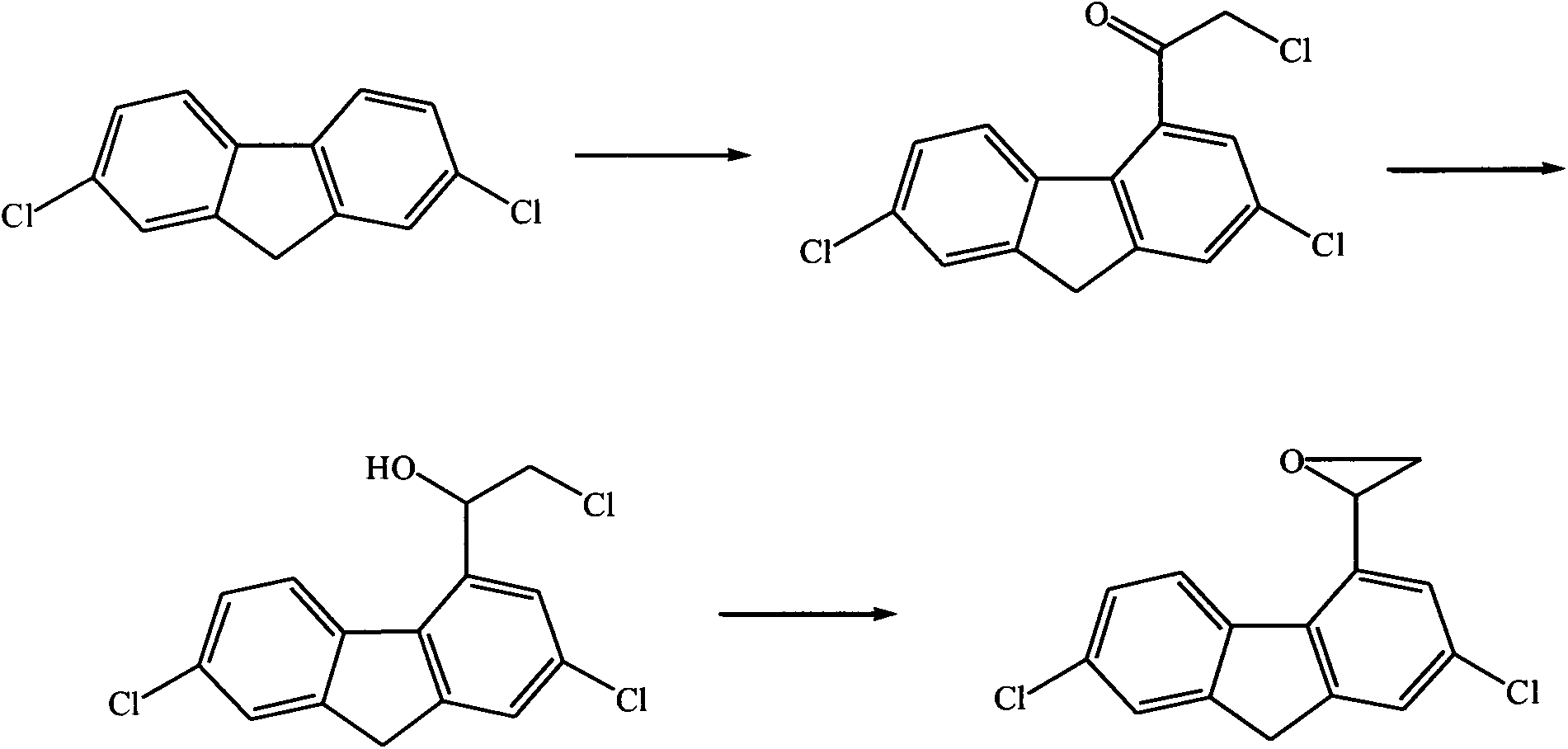
Anti-malaria medical raw material benflumetol intermediate 2,7-dichlorofluorene-4-ethylene oxide synthesis process
A technology of ethylene oxide and dichlorofluorene, applied in 2 fields, can solve the problems such as high price of bromoacetyl bromide, unenvironmental protection, unsuitable for industrialized production, etc., achieves green environmental protection production procedure, great economic and social benefits, and simple equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0021] Embodiment 12, the synthesis of 7-dichlorofluorene-4-oxirane intermediate
[0022] In a 3000L enamel reaction kettle, add 2000L of dichloromethane and anhydrous AlCl 3 25kg, stir and disperse, control the temperature of the ice-salt bath below 5°C, slowly add 160kg of chloroacetyl chloride, and stir for 1.5h for complexation, then slowly add 330kg of 2,7-dichlorofluorene in batches, the process control temperature is within 5°C, After reacting for 2 hours, the temperature was naturally raised to room temperature to continue the reaction for 2 hours until the raw material 2,7-dichlorofluorene was completely reacted as monitored by thin layer chromatography. Continue in the above-mentioned enamel reaction kettle, control the temperature within 10°C, add 140L of 10% NaOH solution, stir the reaction while adding, until the pH value is neutral, remove excess chloroacetyl chloride and neutralize excess AlCl 3 . Continue to add 2.5 kg of potassium borohydride in batches at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com