Metal ion modified nitrogen-containing organic compound for storing hydrogen
A metal ion modification, organic compound technology, applied in the field of hydrogen storage materials, can solve problems such as difficulty in hydrogen absorption, and achieve the effects of large-scale production, simple preparation process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
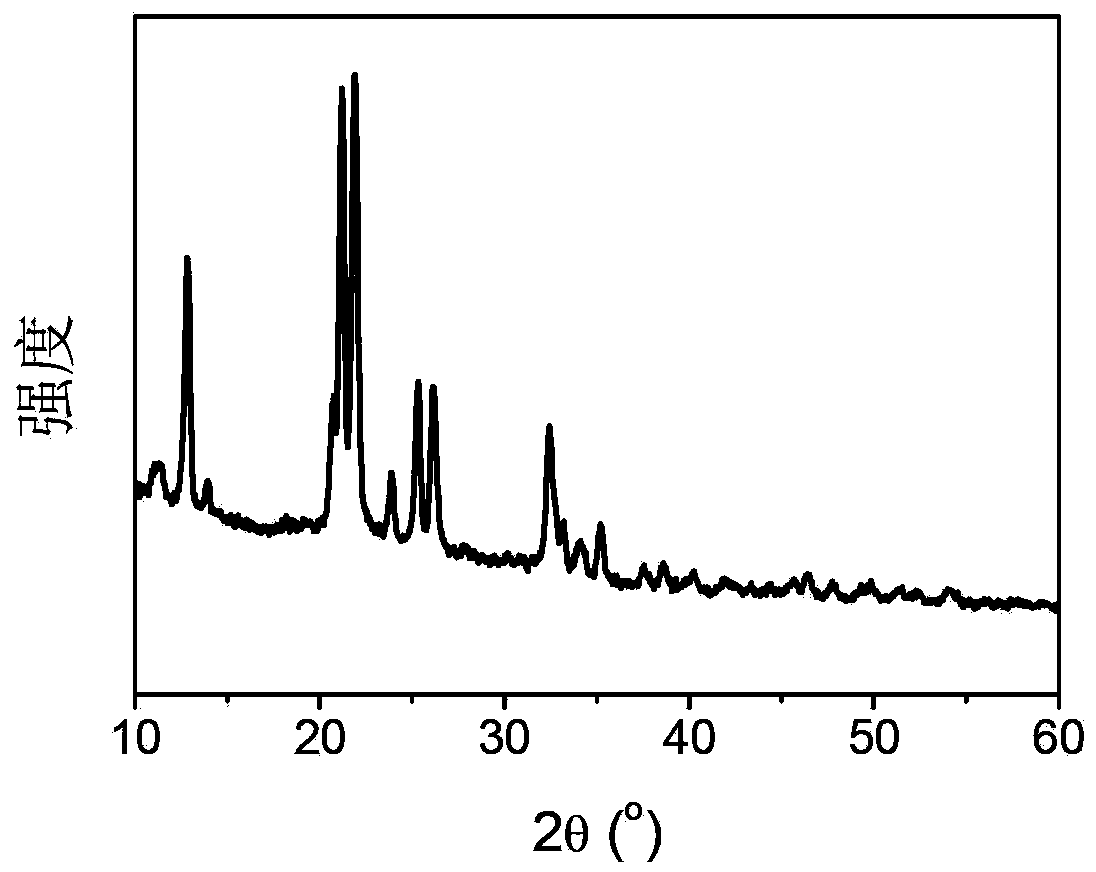
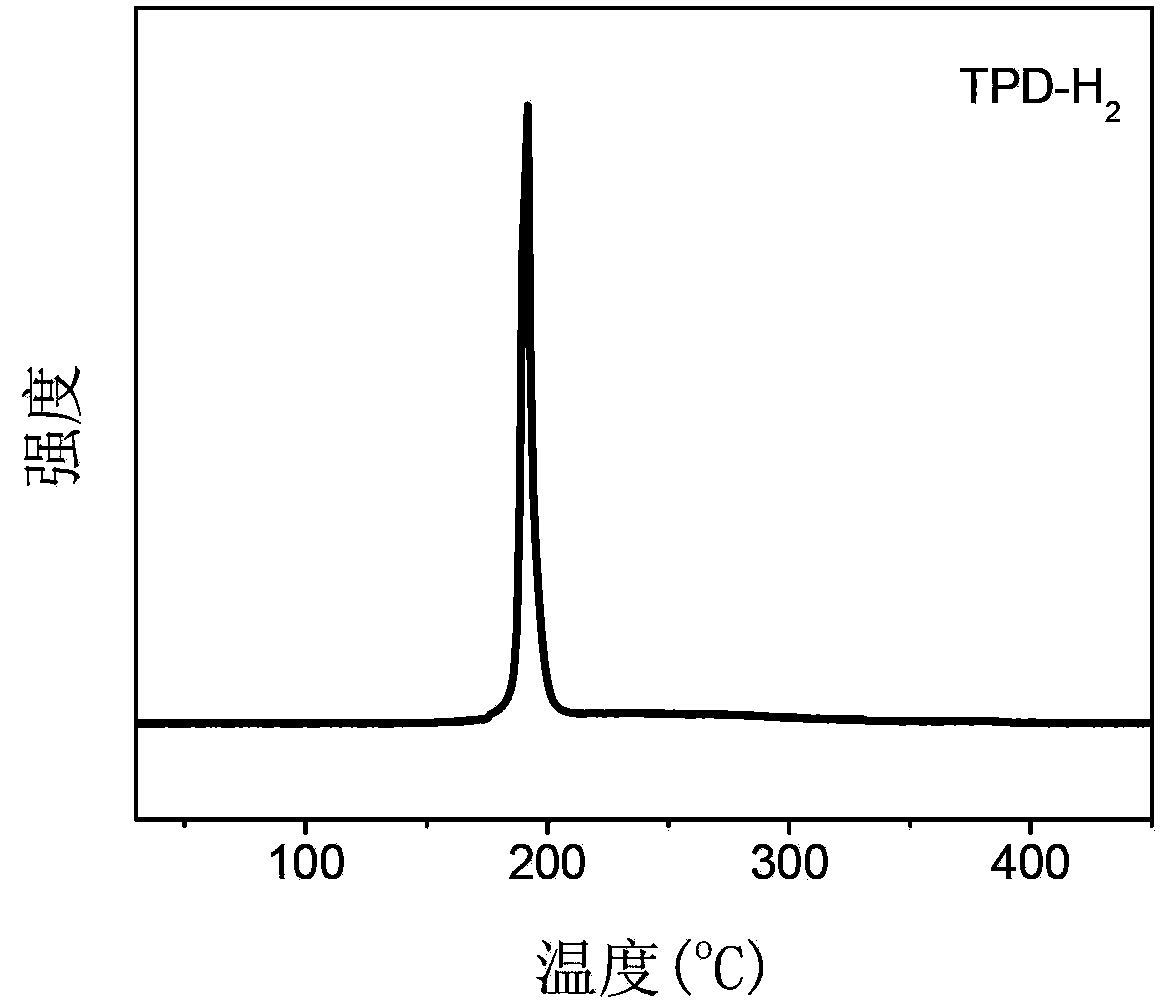
[0055] In a glove box filled with high-purity argon, measure 1 ml of ethylenediamine liquid in a ball mill jar; weigh 0.24 g of lithium hydride, press it into a disc with a diameter of about 1 cm with a manual tablet press, and place the lithium hydride disc The slices were placed on the sample holder installed on the ball mill jar, protected by argon, and mixed by ball milling on a planetary ball mill. 36 hours. Under the condition of cutting off the air, the above-mentioned ball-milled product was taken and fired at 120 degrees centigrade for 24 hours. The X-ray diffraction pattern of the product is obtained as figure 1 As shown, there are only diffraction peaks of the new substance in the solid reaction product, and the existence of the raw material is not seen, indicating that the reaction is carried out completely and there is no reactant remaining. Its temperature-programmed dehydrogenation behavior is as follows figure 2 As shown, hydrogen can be released at 180 deg...
Embodiment 2
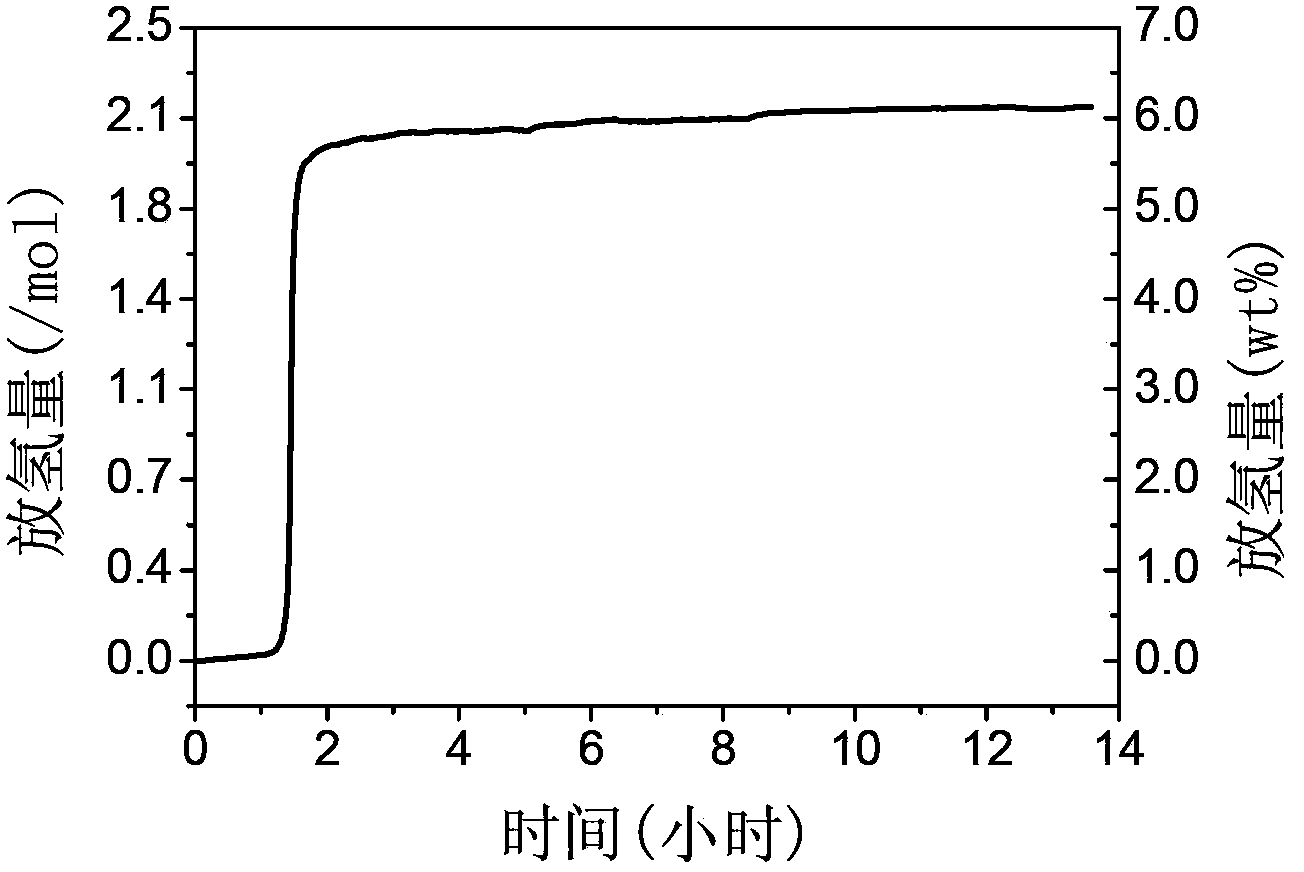
[0057] In a glove box filled with high-purity argon, weigh 0.38 g of acetamidine hydrochloride and 0.2 g of sodium hydride in a ball mill jar, use argon protection, and ball mill and mix on a planetary ball mill. The ball-to-material ratio is 100:1 , the rotation speed is 200 cycles / min, the ball milling temperature is room temperature, and the ball milling time is 6 hours. Under the condition of being isolated from air, the above-mentioned ball milling product was collected. The X-ray diffraction pattern of the product is obtained as Figure 4 As shown, there are only diffraction peaks of sodium chloride and new substances in the solid reaction product, and the existence of raw materials cannot be seen, indicating that the reaction is carried out completely, and there is no reactant residue. Its temperature-programmed dehydrogenation behavior is as follows Figure 5 As shown, hydrogen can be released at 196 degrees Celsius.
Embodiment 3
[0059] In a glove box filled with high-purity argon, weigh 0.36 g of acetamide and 0.15 g of sodium hydride in a ball mill jar, and use argon protection to ball mill and mix them on a planetary ball mill with a ball-to-material ratio of 100:1 and a rotation speed of 200 cycles / minute, the ball milling temperature is room temperature, and the ball milling time is 4 hours. Under the condition of being isolated from air, the above-mentioned ball milling product was collected. The X-ray diffraction pattern of the product is obtained as Figure 6 As shown, there are only diffraction peaks of sodium chloride and new substances in the solid reaction product, and the existence of raw materials cannot be seen, indicating that the reaction is carried out completely, and there is no reactant residue. Its temperature-programmed dehydrogenation behavior is as follows Figure 7 Shown, hydrogen begins to be released around 200 degrees Celsius.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com