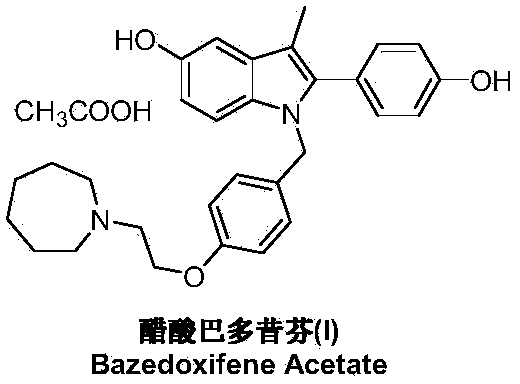
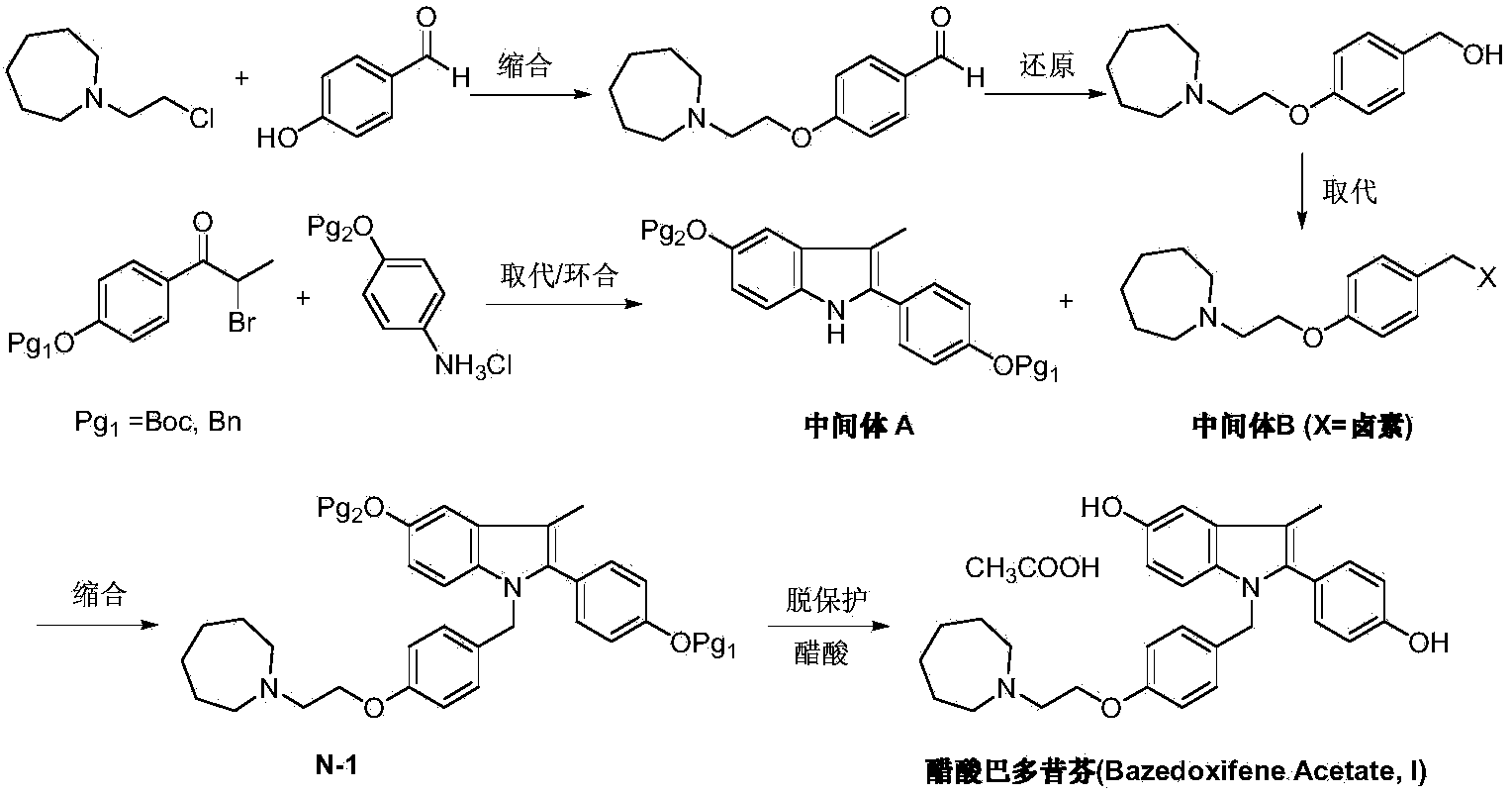
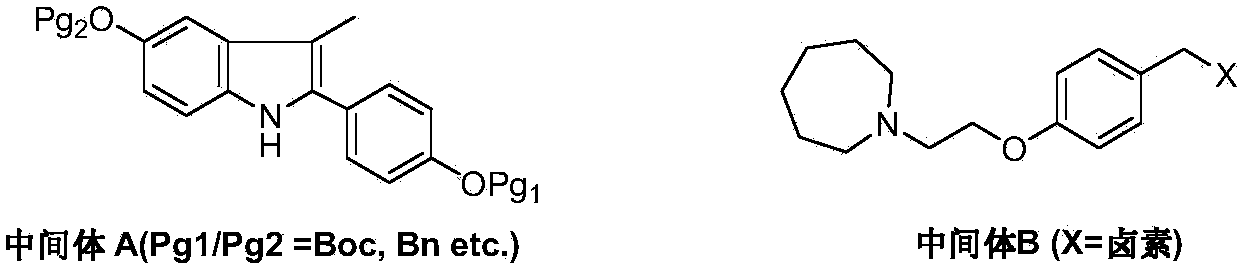Preparation method of bazedoxifene acetate
A technology of bazedoxifene acetate and acetic acid, which is applied in the design of organic synthesis routes and the field of preparation of raw materials and intermediates, can solve the problems of difficulty in obtaining raw materials, many reaction steps, and high pressure on environmental protection, and achieves product yield and product yield. High purity, quick and convenient preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under argon protection, 1-(4-methoxy-phenyl)propanol (II) (1.66g, 10mmol), N-[4-(2-azepane-1 -yl-ethoxy-benzyl)]-N-[4-(methoxyphenyl)]hydrazine (III) (3.69g, 10mmol), triruthenium dodecacarbonyl (0.13g, 0.2mmol), 2 , 2'-bis(diphenylphosphine)biphenyl (0.16 g, 0.3 mmol), crotononitrile (0.67 g, 10 mmol) and 2-methyl-2-butanol 50 mL. Anhydrous zinc chloride (1.36g, 10mmol) was added at room temperature, and after shaking for 5 minutes, the reaction tube was replaced with argon, sealed and placed in a 250W microwave oven, heated to 130°C, irradiated for 3 hours, and the reaction was detected by TLC. The reaction solution was concentrated, dissolved in dichloromethane, washed twice with water, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was recrystallized from ethanol to obtain off-white solid 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-methoxy-phenyl)- 4.2 g of 3-methyl-5-methoxy-1H-indole ...
Embodiment 2
[0035] Under argon protection, 1-(4-benzyloxy-phenyl)propanol (II) (2.42g, 10mmol), N-[4-(2-azepane-1 -yl-ethoxy-benzyl)]-N-[4-(benzyloxyphenyl)]hydrazine (III) (4.45g, 10mmol), triruthenium dodecacarbonyl (0.13g, 0.2mmol), 2 , 2'-bis(diphenylphosphine)biphenyl (0.16 g, 0.3 mmol), crotononitrile (0.67 g, 10 mmol) and cyclopentyl methyl ether 50 mL. Anhydrous zinc chloride (1.36g, 10mmol) was added at room temperature, and after shaking for 5 minutes, the reaction tube was replaced with argon, sealed and placed in a 250W microwave oven, heated to 140°C, irradiated for 3 hours, and the reaction was detected by TLC. The reaction solution was concentrated, dissolved in dichloromethane, washed twice with water, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The crude product was recrystallized from ethanol to obtain off-white solid 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-methoxy-phenyl)- 5.5 g of 3-methyl-5-methoxy-...
Embodiment 3
[0037]Under nitrogen protection and at -78°C, add 1-[4-(2-azepan-1-yl-ethoxy-benzyl)]-2-(4-methoxy-benzene yl)-3-methyl-5-methoxy-1H-indole (IV) (2.5g, 5mmol) and dichloromethane 25mL. Under stirring, a solution of boron tribromide (2.5 g, 10 mmol) in 25 mL of dichloromethane was added dropwise, and the mixture was reacted at room temperature for 10 hours. The reaction was quenched by adding saturated ammonium chloride solution, and the organic layer was separated, dried and concentrated under reduced pressure. The residue was dissolved in 50 mL of ethanol, added with glacial acetic acid (0.3 g, 5 mmol), stirred at room temperature and crystallized at 5° C. for 3 hours to obtain 2.25 g of bazedoxifene acetate (I), with a yield of 84.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com