Preparation method, products and application of Sb2O5 adsorbent containing doped metal ions
A metal ion and ion doping technology, applied in chemical instruments and methods, other chemical processes, inorganic chemistry, etc., can solve the problems of large-scale preparation and difficulty in obtaining unfavorable materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
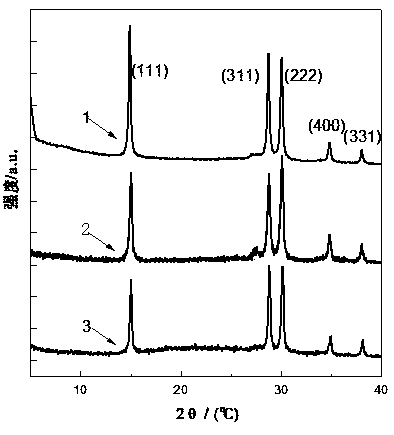
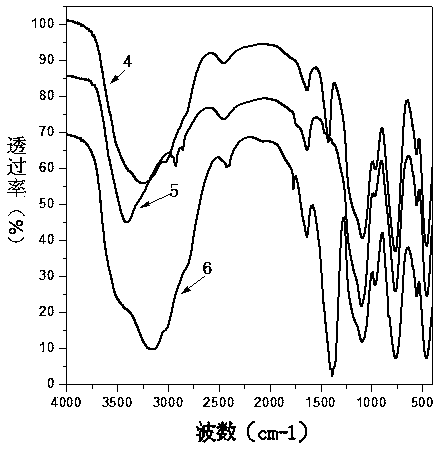
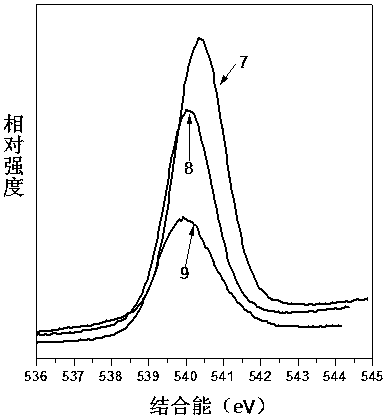
[0047] Step 1: 4.56g of anhydrous SbCl 3Dissolve 4.46mL of TEOS in 20mL of anhydrous ethylene glycol, configure a solution containing Si(IV) / Sb(III) in a 250mL three-necked flask, and the flask is equipped with electromagnetic stirring, oil bath heating and water cooling to condense and reflux Tube. Heat in an oil bath to make the solution reach the set reaction temperature of 60°C.
[0048] Step 2: Keep the above set temperature at 60°C, slowly add 30% H at a rate of 5-10 drops / min 2 o 2 Solution 20mL. A slight bumping phenomenon occurred at the beginning of the dropwise addition, but disappeared as the dropwise addition progressed. During the dropwise addition of hydrogen peroxide, a colorless transparent colloidal solution was first generated, and then a white precipitate appeared in the reaction solution.
[0049] Step 3: After the dropwise addition, set the reaction temperature to 80°C, turn on the ultraviolet lamp (254nm) placed outside the flask, and turn off the u...
Embodiment 2
[0059] With reference to Example 1 in this embodiment, H in step 2 2 o 2 The addition amount of the solution is adjusted to 6mL, and other operations are the same as in Comparative Example 1, and the material preparation is carried out to obtain a solution containing Si / Sb 2 o 5 the adsorbent.
[0060] Add 30% H dropwise 2 o 2 The oxidation rate of Sb(III)→Sb(V) in 6mL of the solution was only 64%, which indicated that a higher dosage of hydrogen peroxide was beneficial to obtain a higher oxidation rate. The Sr ion adsorption performance of material is measured, under the state that the initial concentration of Sr ion is 10mg / L, the material of the present embodiment is to the distribution coefficient K of Sr ion d-sr It was 39754186mg / L, compared with Comparative Example 1, the adsorption performance decreased.
Embodiment 3
[0062] Step 1: 4.56g of anhydrous SbCl 3 and 4.46mL of TEOS were dissolved in 20mL of anhydrous ethylene glycol, and then the solution was added to a 250mL three-necked flask equipped with electromagnetic stirring, oil bath heating, and a water-cooled condensing reflux tube. Heat the oil bath to make the solution reach the set reaction temperature of 60°C
[0063] Step 2: Keep the above set temperature at 60°C, and slowly add 30% H2 to the three-necked flask at a rate of 5-10 drops / min. 2 o 2 Solution 20mL. A slight bumping phenomenon occurred at the beginning of the dropwise addition, but disappeared as the dropwise addition progressed. During the dropwise addition of hydrogen peroxide, a colorless transparent colloidal solution was first generated, and then a white precipitate appeared in the reaction solution.
[0064] Step 3: After the dropwise addition, set the reaction temperature to 80°C, turn on the ultraviolet lamp (254nm) placed outside the flask, and turn off th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com