Method for detecting complete degradation product of low molecular weight heparin based on post-column derivatization
A low-molecular-weight heparin, detection method technology, applied in the direction of measurement device, material separation, analysis material, etc., can solve the problems affecting the accuracy and repeatability of analysis, chromatographic separation interference, affecting analysis results, etc., to achieve great practical value, analysis High degree of automation and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The following will clearly and completely describe the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
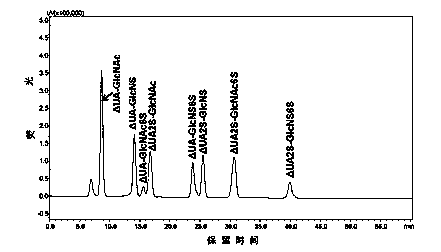
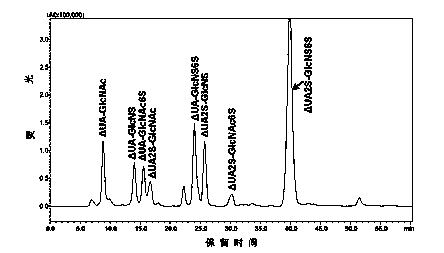
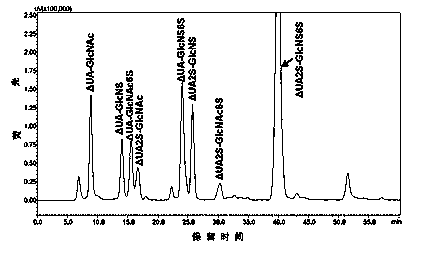
[0026] A method for detecting a complete enzymatic hydrolysis product of low molecular weight heparin is provided, comprising the steps of:
[0027] (1) Low molecular weight heparin sodium was degraded with heparinase I, II and III at 25°C for 48 h, the degradation product was filtered by ultrafiltration membrane, dried under vacuum and reduced pressure, and then prepared into 1 mg / mL preparation with deionized water. test solution.
[0028] (2) Preparation of the first mobile phase: Dissolve tetrabutylammonium bisu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com