Use of trypsin
A technology for trypsin and uses, applied in the field of trypsin, can solve problems such as toxic effects, destruction of cell components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
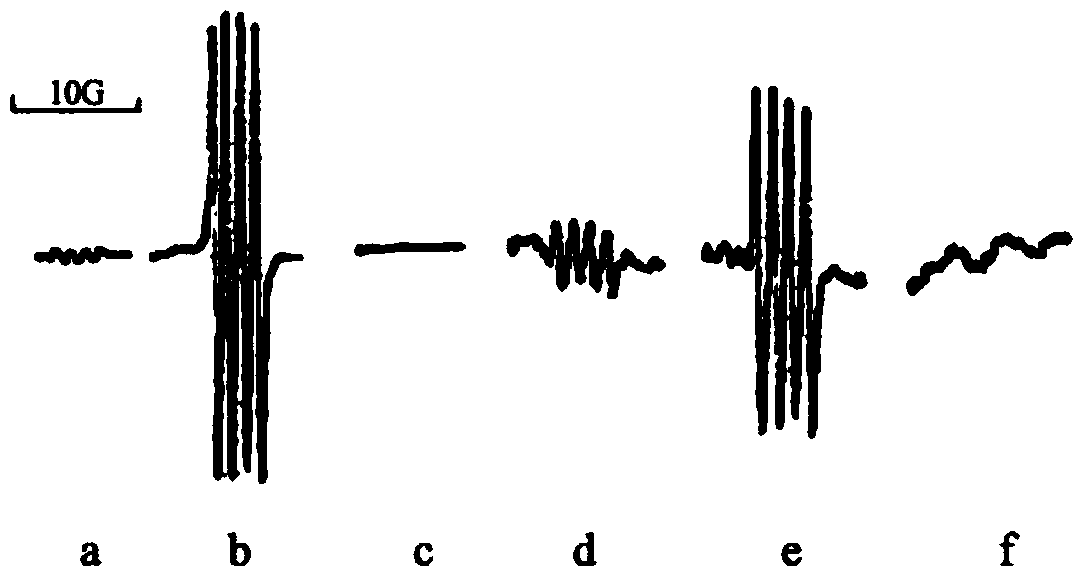
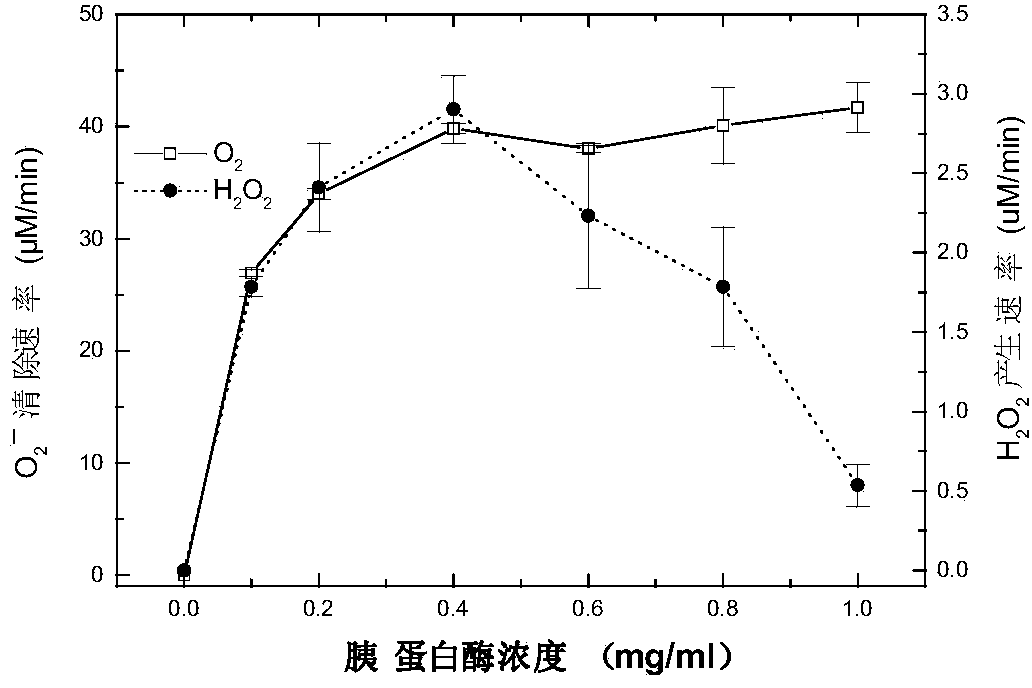
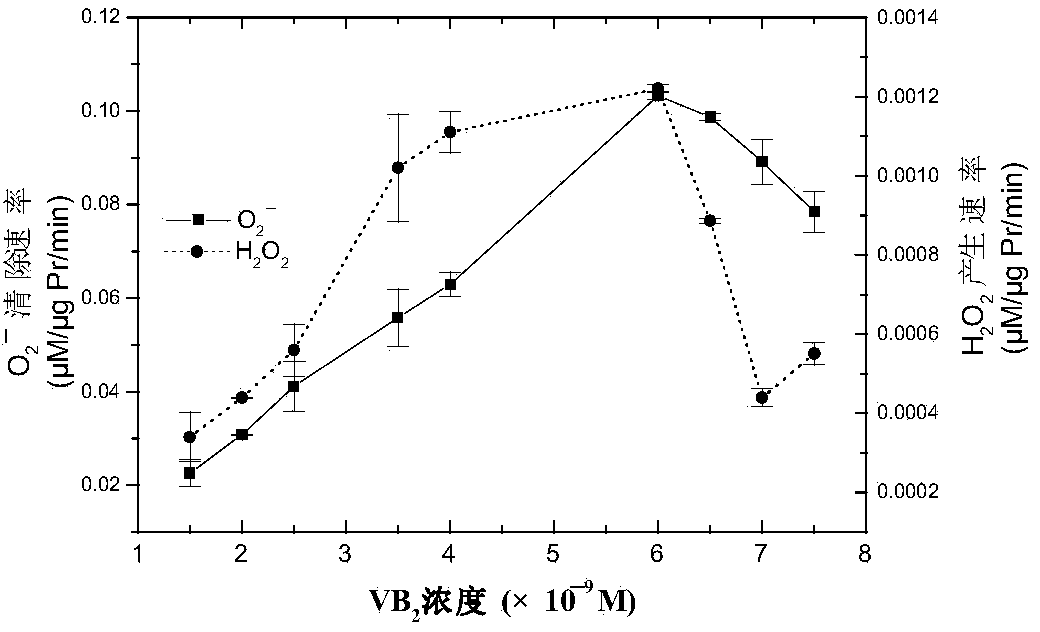
[0025] In this example, the application of trypsin in scavenging free radicals.
[0026] 1. Materials and methods
[0027] 1. Materials
[0028] Escherichia coli wild-type strain MG1655 was donated by Professor Imlay of Illinois State University. Streak inoculate the dry powder of the above-mentioned strains stored in freeze-dry on LB plates, culture at 37°C for more than 16 hours, pick a single colony into the LB liquid medium, and cultivate at 37°C until the concentration of the bacterial solution is about 10 9 cells / ml, glycerol -20 ℃ to save the strains, for later use.
[0029] 2. Trypsin treatment
[0030] Trypsin (500U / mg) was purchased from Amersco, take 100mg.ml -1 Trypsin was added to O 2 ·- Incubate the resulting mixed system at 37°C for 30min, then add 25μl of 10mg.ml -1 Soybean trypsin inhibitor terminates the reaction.
[0031] 3. Sample processing
[0032] Sample with 0.5mM Cu 2+ Either incubate with 25mM EDTA for 0.5h or incubate with 1mM DDC at 28°C for...
Embodiment 2
[0053] The application of trypsin in anti-aging (delaying aging) in this example.
[0054] The tumor mice with no significant difference in the number of voluntary activities were selected and divided into two groups, 10 in each group. The control group was intragastrically given 10 mL / kg of normal saline according to the weight of the mice, and the experimental group was given intravenous injection of normal saline (10 mL / kg) containing 0.6 mg / mL trypsin according to the weight of the mice, and the survival time of the mice was recorded. The results showed that the mice in the control group died within 1 week, and the survival period of the mice in the trypsin treatment group was extended to 8 weeks.
Embodiment 3
[0056] In this example, the application of trypsin in anti-tumor (inhibition of tumor cell proliferation activity).
[0057] Hela cells in the logarithmic growth phase were collected, and the experimental group was treated with trypsin (0.6 mg / ml), and the control group was added with 200 μL of bovine serum culture medium, and the temperature was 5% CO at 37°C. 2 and fully saturated humidity CO 2 Cultivate in the incubator for 24-48 hours, and detect the cell survival rate by MTT method. The results showed a 30 percent decrease in tumor cell survival in the trypsin-treated group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com