Method of preparing 6-deoxy-L-talose
A technology for talose and rhamnose, which is applied in the field of synthesizing 6-deoxy-L-talose, can solve the problems of high price, cumbersome steps, few commercially available sources, etc., and achieves avoiding pollution, improving yield, simplifying Effects of compositing and post-processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
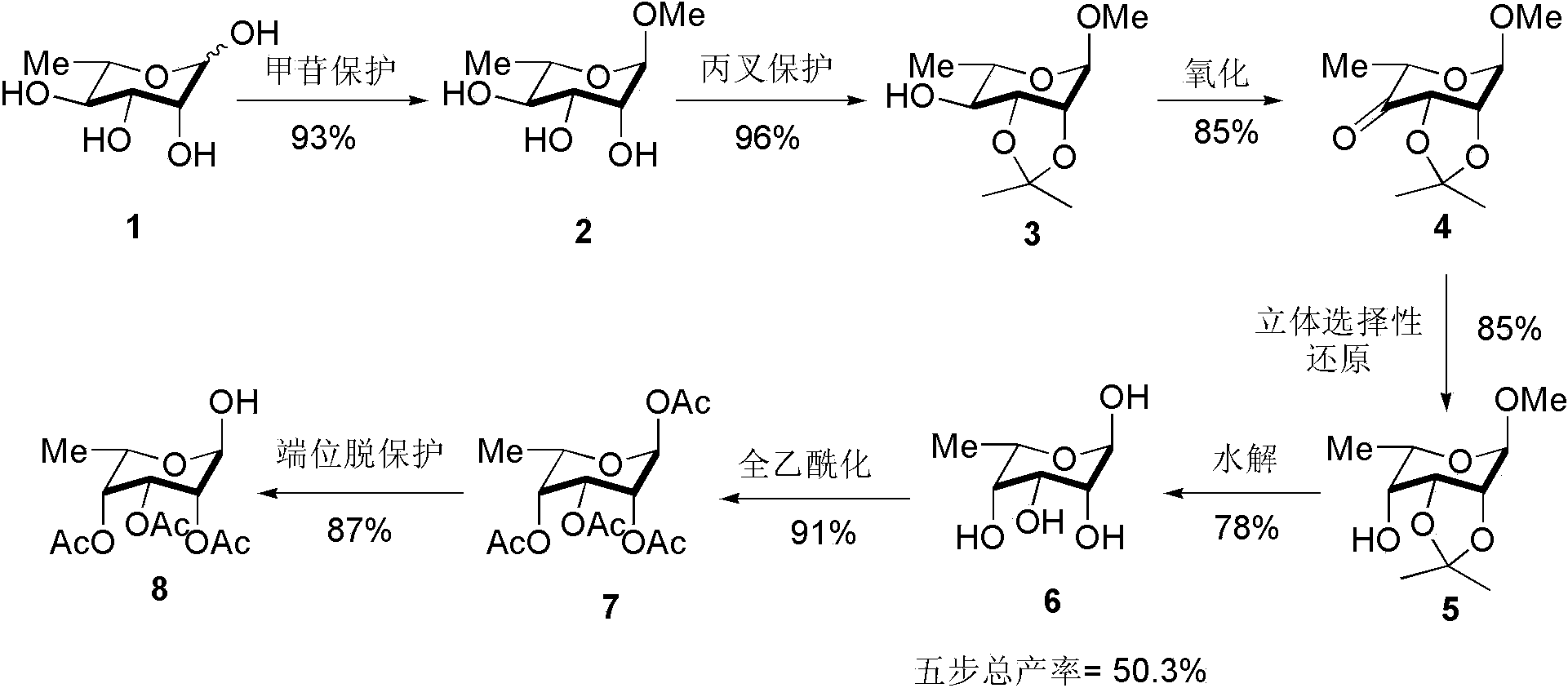
[0019] Step 1: Methyl-6-L-Rhamnose 2 Synthesis of: Dissolve L-rhamnose (40 g, 244 mmol) in methanol (400 mL), add H + Type cation exchange resin (40 g, 103 mmol) was stirred and refluxed for 12 h, then the resin was filtered off under reduced pressure, and the filtrate was concentrated to obtain 40 g of yellow oily glycoside-protected L-rhamnose, yield: 93%.
[0020] Step 2: Methyl-6-deoxy-2,3- O -Isopropylidene-6-L-rhamnoside 3 Synthesis of glycoside-protected L-rhamnose (40 g, 224.7 mmol) was dissolved in acetone (85 mL, 1123 mmol) and 2,2-dimethoxypropane (138 mL, 1123 mmol) mixed solution, Add p-toluenesulfonic acid (1.548 g, 9.0 mmol), and after stirring at room temperature for 2 h, wash with solid NaHCO 3 Neutralize p-toluenesulfonic acid until the pH of the system is greater than 7, remove the solvent under reduced pressure, and then separate by dry column chromatography (dichloromethane:methanol = 30:1) to obtain 47 g of a light yellow oily product, with a yield of ...
Embodiment 2
[0027] Step 1: Methyl-6-L-Rhamnose 2 Synthesis of: Dissolve L-rhamnose (40 g, 244 mmol) in methanol (400 mL), add H + Type cation exchange resin (40 g, 103 mmol) was stirred and refluxed for 12 h, then the resin was filtered off under reduced pressure, and the filtrate was concentrated to obtain 40 g of yellow oily glycoside-protected L-rhamnose, yield: 93%.
[0028] Step 2: Methyl-6-deoxy-2,3- O -Isopropylidene-6-L-rhamnoside 3 The synthesis of glycoside-protected L-rhamnose (20 g, 112.3 mmol) was dissolved in acetone (41 mL, 562 mmol) and 2,2-dimethoxypropane (69 mL, 562 mmol) mixed solution, Add concentrated sulfuric acid (650 μL, 12.2 mmol), stir at room temperature for 2 h, then wash with solid NaHCO 3 Neutralize p-toluenesulfonic acid until the pH of the system is greater than 7, remove the solvent under reduced pressure, and then separate by dry column chromatography (dichloromethane: methanol = 30:1) to obtain 23 g of a light yellow oily product, with a yield of 86%...
Embodiment 3
[0035] Step 1: Methyl-6-L-Rhamnose 2 Synthesis of: Dissolve L-rhamnose (40 g, 244 mmol) in methanol (400 mL), add H + Type cation exchange resin (40 g, 103 mmol) was stirred and refluxed for 12 h, then the resin was filtered off under reduced pressure, and the filtrate was concentrated to obtain 40 g of yellow oily glycoside-protected L-rhamnose, yield: 93%.
[0036] Step 2: Methyl-6-deoxy-2,3- O -Isopropylidene-6-L-rhamnoside 3 Synthesis of glycoside-protected L-rhamnose (40 g, 224.7 mmol) was dissolved in acetone (85 mL, 1123 mmol) and 2,2-dimethoxypropane (138 mL, 1123 mmol) mixed solution, Add p-toluenesulfonic acid (1.548 g, 9.0 mmol), and after stirring at room temperature for 2 h, wash with solid NaHCO 3 Neutralize p-toluenesulfonic acid until the pH of the system is greater than 7, remove the solvent under reduced pressure, and then separate by dry column chromatography (dichloromethane:methanol = 30:1) to obtain 47 g of a light yellow oily product, with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com