Tenofovir disoproxil fumarate tablets allowing direct powder compression and preparation method thereof
A technology for tenofovir fumarate and pyrifurate tablets is applied in the field of tablets and their preparation, and can solve the problems of high viscosity of tenofovir disoproxil fumarate, unfavorable stability and impurity content, Affecting drug efficacy and other issues, achieving the effect of being suitable for industrial-scale operation, good dissolution, and small difference in tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] Example general method:
[0028] (1) Weigh microcrystalline cellulose, talc and component A with a limited weight percentage and mix them uniformly to obtain a premixed auxiliary material.
[0029] (2) Add tenofovir disoproxil fumarate to the premixed excipients in several times. After each addition of the main drug should be mixed with the premixed excipients, continue to add the main drug until the main drug is added. Add all tenofovir disoproxil fumarate and mix well. After the end of the divided addition, a mixture was obtained.
[0030] (3) The mixture was placed in a ZP-35 II tableting machine, and the powder was directly compressed into 1000 tablets.
[0031] The whole process is done in a dry environment.
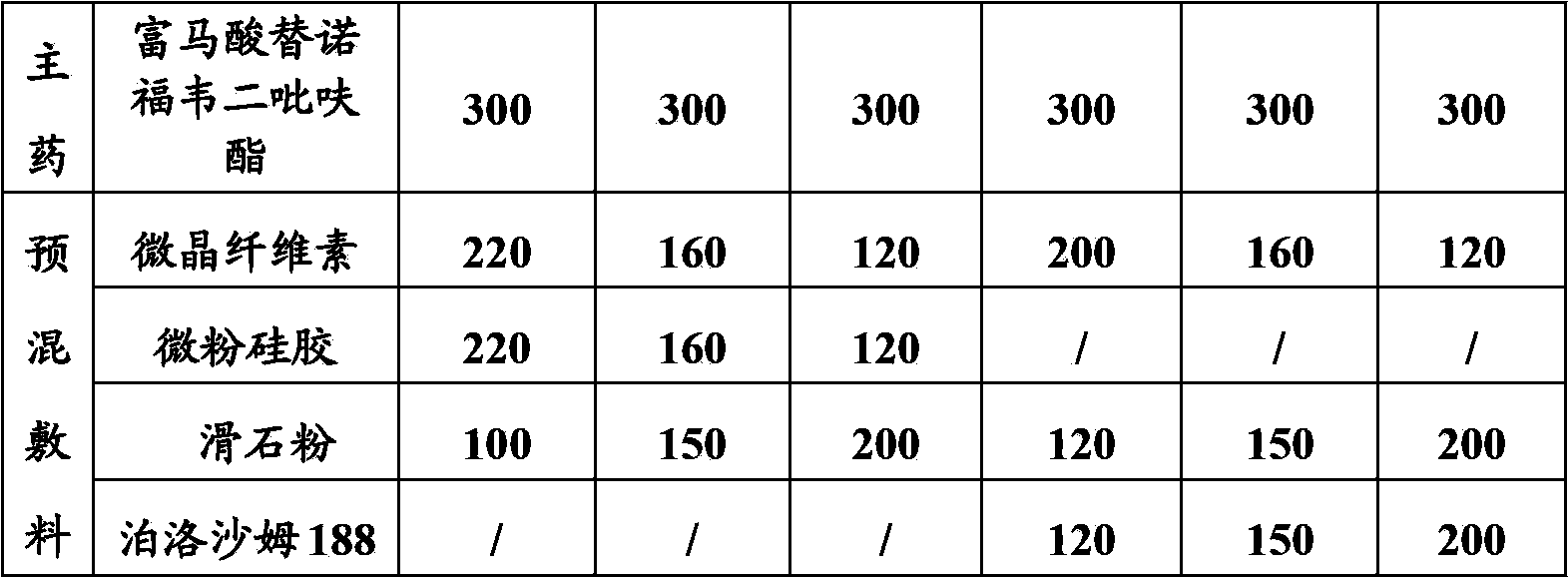
[0032] The prescription amounts of the main drug and the premixed adjuvant in each example are listed in Table 1 below.
[0033] Table 1—the prescription content (g) of main drug and premixed adjuvant in each embodiment
[0034]
[0035]
Embodiment 7
[0037] Example 7 is a comparative example with reference to the prior art, which is the wet granulation method described in US Pat. No. 5,935,946.
[0038] Main Drugs and Excipients:
[0039]
[0040] The preparation method includes the following steps:
[0041] (1) Take the stated amounts of tenofovir disoproxil fumarate, lactose monohydrate, pregelatinized starch, croscarmellose sodium and purified water.
[0042] (2) Mix tenofovir disoproxil fumarate, lactose monohydrate, pregelatinized starch, and croscarmellose sodium evenly, then add purified water to prepare soft materials, and use a 20-mesh sieve to wet-process After drying at 50°C for 5 hours, granulate with a 20-mesh sieve.
[0043] (3) Adding additional lactose monohydrate, croscarmellose sodium and magnesium stearate to the above dry granules, and mixing them uniformly to obtain a mixture.
[0044] (4) Put the mixture in a tablet press and press it into 1000 tablets.
[0045] Comparative Results
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com