Method for recovering copper, cobalt and nickel from copper and nickel slag
A copper-nickel slag and copper recovery technology, applied in the direction of improving process efficiency, can solve the problem of high cost and achieve the effect of high economic value and social benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
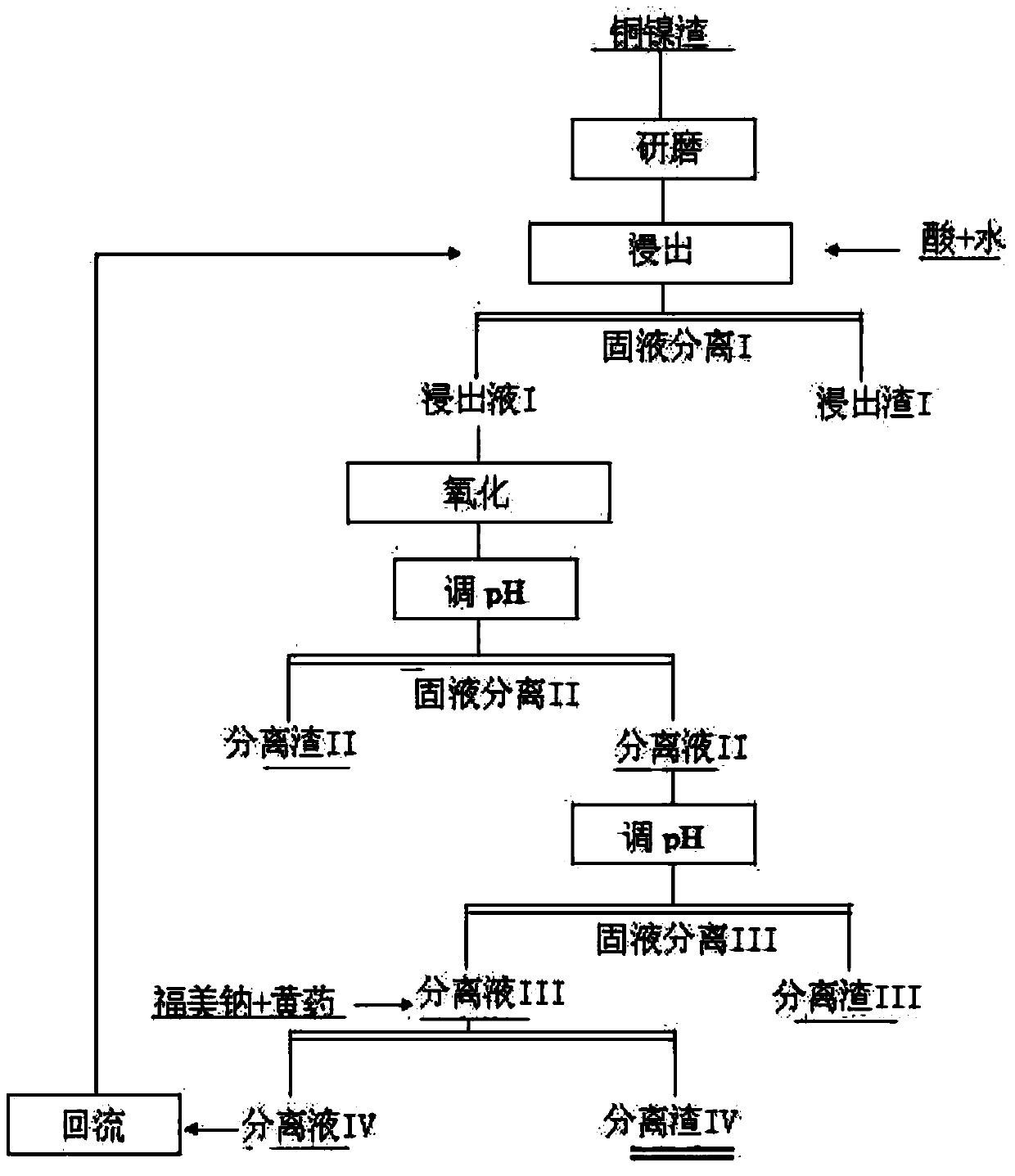
[0029] a. Use 100g of ground 80-mesh copper waste slag ore powder to leach soluble matter by step-by-step concentration method. In the first step, water and concentrated acid are added in proportion to the leaching process. The acid concentration of the pulp is 50% and the temperature is 60°C. Under the leaching for 60 minutes; the second step of leaching uses a pulp acid concentration of 15%, with the reflux waste liquid as the leaching solution, the leaching time is 80 minutes, and the leaching temperature is normal temperature, and the mixture is separated from the solid and liquid to obtain the leaching residue I and the leaching solution I ( Cu=151mg / L, Ni=125mg / L, Co=69mg / L);
[0030] b. Oxygen oxidation is passed into 521mL leach solution I, and low-valent iron ions are oxidized into high-valent ions, and an appropriate amount of copper-nickel slag is added to the oxidized acid solution to adjust the pH value to about 3, and high-valent iron is precipitated in the form o...
Embodiment 2
[0036] a. Use 100g of ground 80-mesh copper waste slag slag powder to leach soluble matter using the step-by-step concentration method. In the first step, water and concentrated acid are added in proportion to the pulp acid concentration of 40% and the temperature is 70°C. 80min under leaching; the second step leaching uses a pulp acid concentration of 15%, using the reflux waste liquid as the leaching solution, the leaching time is 80min, the leaching temperature is normal temperature, and the mixture is separated from the solid and liquid to obtain the leaching slag I and the leaching solution I ( Cu=163mg / L, Ni=155mg / L, Co=88mg / L);
[0037]b. Oxygen oxidation is passed into 534mL leachate I, low-priced iron ions are oxidized into high-valent ions, and an appropriate amount of copper-nickel slag is added to the oxidized acid solution to adjust the pH value to about 3, and high-valent iron is precipitated in the form of ferric hydroxide. Separation slag II and separation liqu...
Embodiment 3
[0043] a. Use 100g of ground 80-mesh copper waste slag ore powder to leach soluble matter using the step-by-step concentration method. In the first step, water and concentrated acid are added in proportion to the leaching. The acid concentration of the pulp is 60% and the temperature is 40°C. 50min under leaching; the second step leaching uses ore pulp with an acid concentration of 15%, the reflux waste liquid is used as the leaching solution, the leaching time is 90min, and the leaching temperature is normal temperature, and the mixture is separated into solid and liquid to obtain leaching residue I and leaching solution I ( Cu=146mg / L, Ni=115mg / L, Co=75mg / L);
[0044] b. Oxygen oxidation is passed into 505mL leachate I, low-priced iron ions are oxidized into high-valent ions, and an appropriate amount of copper-nickel slag is added to the oxidized acid solution to adjust the pH value to about 3, and high-valent iron is precipitated in the form of ferric hydroxide. Separation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com