Method for recycling fluorine-containing waste gas in phosphorus chemical industry production
A phosphorus chemical and exhaust gas technology, which is applied in the direction of silicon oxide, silicon dioxide, fluorine/hydrogen fluoride, etc., can solve the problems of unfavorable industrial application, and achieve the effects of easy industrial application, low pollution, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
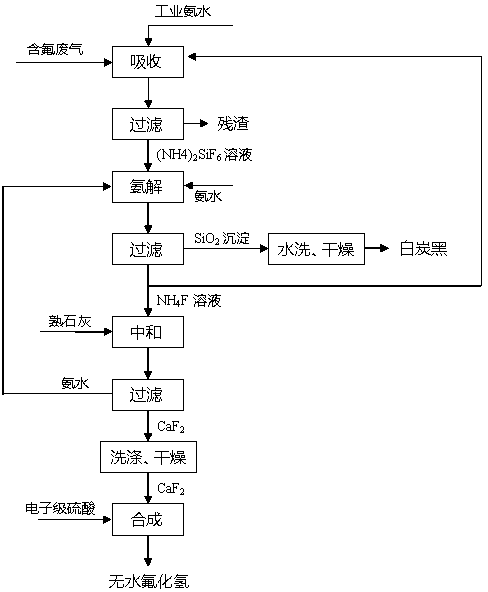
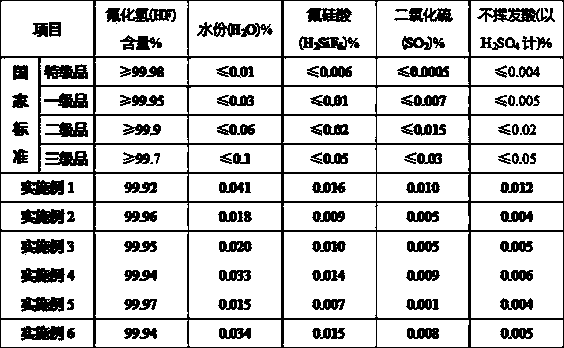
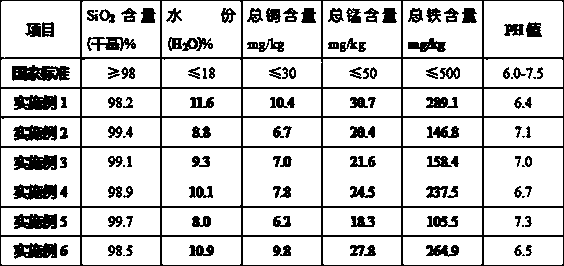
[0024] Add a sufficient amount of industrial ammonia water into the absorption tower to absorb fluorine-containing waste gas, control the reaction temperature in the tower to 45°C, and the reaction time is 1 hour, then filter the residue to obtain 937kg of ammonium fluorosilicate solution with a concentration of 12%; Add 3 kg of ammonia water with a concentration of 11% to the acid solution, . The ammonolysis reaction was carried out under the condition of 0, and the reaction was performed for 1 hour. After filtration, 334kg of silica precipitate and 685kg of ammonium fluoride solution were obtained. After the silica precipitate was washed with water, it was dried at a temperature of 110°C for 40 minutes to obtain a surface area of 750㎡ / g of white carbon black product 328kg; 685kg of ammonium fluoride solution (accounting for 40%) is returned to the absorption system for recycling to absorb fluorine-containing waste gas, and the rest is neutralized with 928kg of industrial g...
Embodiment 2
[0026] Add a sufficient amount of industrial ammonia water into the absorption tower to absorb fluorine-containing waste gas, control the reaction temperature in the tower to 50°C, and the reaction time to 2 hours, then filter the residue to obtain 1048kg of ammonium fluorosilicate solution with a concentration of 15%; Add 4kg of ammonia water with a concentration of 15% to the acid solution, . Under the condition of 5, carry out ammonolysis reaction, reaction 1 . Filtrate after 5 hours, obtain silica precipitation 368kg and ammonium fluoride solution 739kg, silica precipitation obtains the white carbon black product 362kg that surface area is 794㎡ / g under the condition of 115 ℃ of temperature after washing; 739kg of ammonium fluoride solution (accounting for 40%) is returned to the absorption system for recycling to absorb fluorine-containing waste gas, and the rest is neutralized with 985kg of industrial-grade slaked lime, the reaction temperature is controlled at 30°C, and ...
Embodiment 3
[0028]Add a sufficient amount of industrial ammonia water into the absorption tower to absorb fluorine-containing waste gas, control the reaction temperature in the tower to 55°C, and the reaction time to 3 hours, then filter the residue to obtain 1027kg of ammonium fluorosilicate solution with a concentration of 20%; Add 3kg of ammonia water with a concentration of 18% to the acid solution, . Ammonolysis reaction was carried out under the condition of 0, and after 2 hours of reaction, it was filtered to obtain 362kg of silica precipitate and 714kg of ammonium fluoride solution. The silica precipitate was washed with water and dried for 45 minutes at a temperature of 120°C to obtain a surface area of 805㎡ / g of white carbon black product 357kg; 714kg of ammonium fluoride solution (accounting for 45%) is returned to the absorption system for recycling to absorb fluorine-containing waste gas, and the rest is neutralized with 928kg of industrial grade slaked lime, and the reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com