Co-precipitation preparation method of composite oxide denitration catalyst
A denitration catalyst and composite oxide technology, which is applied in the field of nitrogen oxide reduction catalyst preparation, can solve the problems of low NOx removal rate and inapplicability, and achieve the effects of increasing specific surface area, improving denitration activity and reducing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
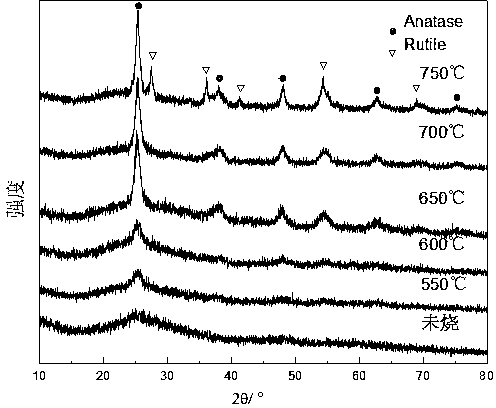
Image
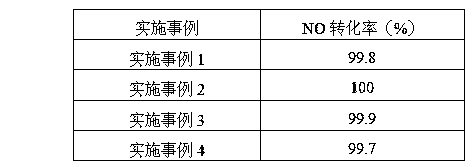
Examples
Embodiment 1
[0019] 1) Preparation of catalyst precursor Preparation: Weigh 166.0g of NaSiO under constant temperature conditions of 60-100°C 3 9H 2 O, 112.0gTiCl 4 , 83.0gAlCl 3 ·6H 2 O and 3.0g ammonium metavanadate, 5.1g ammonium tungstate, 30.0g chromium nitrate, 13.6g nickel nitrate, 5.1g manganese chloride.
[0020] Then add water or hydrogen peroxide respectively to make NaSiO 3 9H 2 O, titanium sulfate aqueous solution, AlCl 3 ·6H 2 O aqueous solution, ammonium vanadate hydrogen peroxide aqueous solution, ammonium tungstate aqueous solution, nickel nitrate aqueous solution, cobalt nitrate aqueous solution, chromium nitrate aqueous solution and manganese chloride aqueous solution.
[0021] Mix the above solutions, add dropwise sodium silicate nonahydrate aqueous solution under stirring condition, after dark green uniform precipitation appears, use H 2 SO 4 Aqueous solution or NH 3 ·H 2 O to adjust the pH of the precipitation system to 8-9, after stirring, let it stand for...
Embodiment 2
[0025] Prepared in the same manner as in Example 1. The difference is: weigh 249.0gNaSiO 3 9H 2 O, 98.0gTiCl 4 , 27.7gAlCl 3 ·6H 2O and 3.0g ammonium metavanadate, 5.1g ammonium tungstate, 30.0g chromium nitrate, 8.2g cobalt nitrate.
[0026] Analysis of TiO in prepared catalyst 2 The content is 35%wt%, SiO 2 45%wt%, Al 2 o 3 5%wt%, V 2 o 5 2%wt%, WO 3 4%wt%, Cr 2 o 3 5%wt%, Co 2 o 3 It is 4%wt%.
Embodiment 3
[0028] Prepared in the same manner as in Example 1. The difference is: weigh 138.3gNaSiO 3 9H 2 O, 140.0gTiCl 4 , 55.4gAlCl 3 ·6H 2 O and 3.8g ammonium metavanadate, 6.4g ammonium tungstate, and 8.1g manganese chloride were used to prepare the catalyst by co-precipitation.
[0029] Analysis of TiO in prepared catalyst 2 Content is 50%wt%, SiO 2 25%wt%, Al 2 o 3 10%wt%, V 2 o 5 2.5%wt%, WO 3 5%wt%, MnO 2 5%wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com