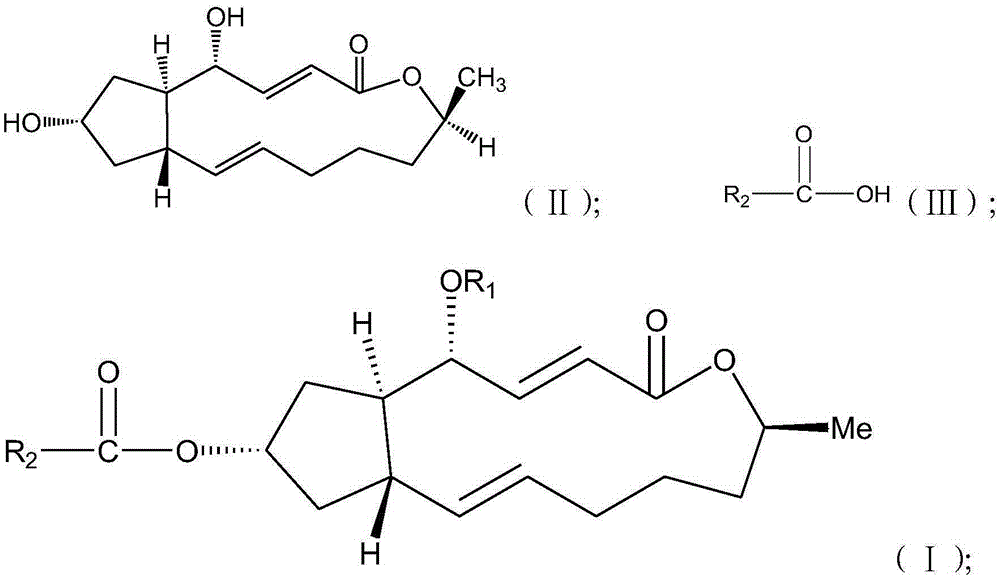
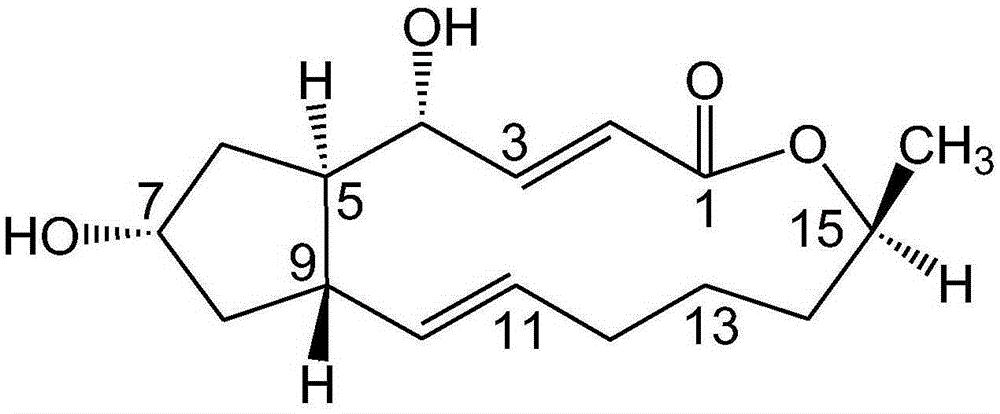
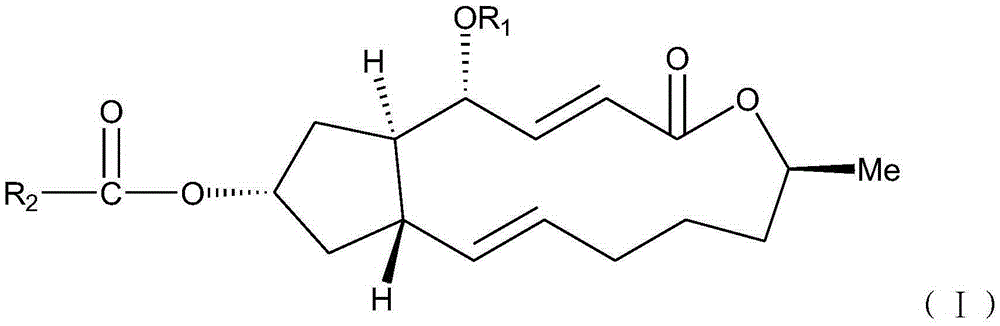
Brefeldin A ester derivatives and their preparation and application
A Brefeldin and Brefeldin technology, which is applied in the directions of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of short plasma half-life, poor water solubility, and unsatisfactory, and achieve high efficiency The effect of curative effect, simple method and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 7-O-acetyl-BFA (I-1), 4,7-O-diacetyl-BFA (I-2)
[0035]
[0036] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round-bottomed flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol), and start stirring; then add acetic acid (45mg, 0.75mmol), EDC.HCl (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) (205mg, 108mmol), DMAP (4-dimethylaminopyridine) (22mg, 0.18mmol), at 40°C The reaction was heated for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Sodium sulfate was dried, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P=1:5 to obtain compound (I-1) (Rf=0.2, yield 42.50%) an...
Embodiment 2
[0040] Example 2: Preparation of 7-O-(benzoic acid) acyl-BFA (I-3), 4,7-O-di(benzoic acid) acyl-BFA (I-4)
[0041]
[0042] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round-bottomed flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol), and start stirring; then add benzoic acid (91.5mg, 0.75mmol), EDC .HCl (205mg, 108mmol), DMAP (22mg, 0.18mmol), heated at 40°C for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Sodium sulfate was dried, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P=1:5 to obtain compound (I-3) (Rf=0.2, yield 46.7%) and Compound (I-4) (Rf=0.8 yield, 29.6%).
[0043] Compound Characterization...
Embodiment 3
[0046] Example 3: Preparation of 7-O-(p-fluorobenzoic acid) acyl-BFA (I-5), 4,7-O-di(p-fluorobenzoic acid) acyl-BFA (I-6)
[0047]
[0048] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round bottom flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol), and start stirring; then add 4-fluorobenzoic acid (105mg, 0.75mmol) , EDC.HCl (205mg, 108mmol), DMAP (22mg, 0.18mmol), heated at 40°C for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Sodium sulfate was dried, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developer E / P=1:5 to obtain compound (I-5) (Rf=0.2, yield 36.4%) and Compound (I-6) (Rf=0.8, yield 20.0%).
[0049] Compound Char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com