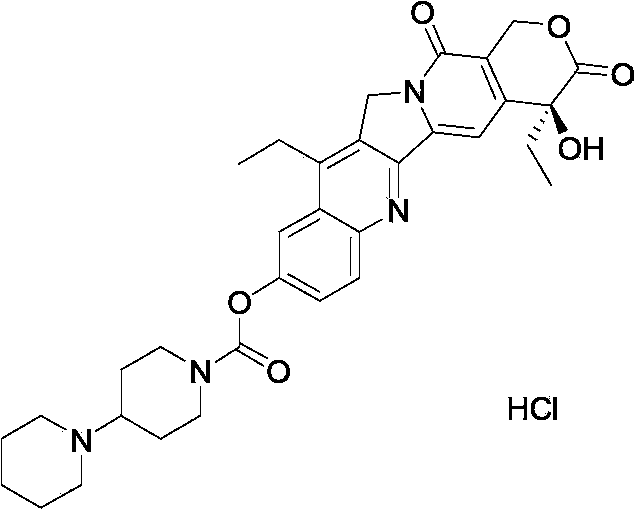
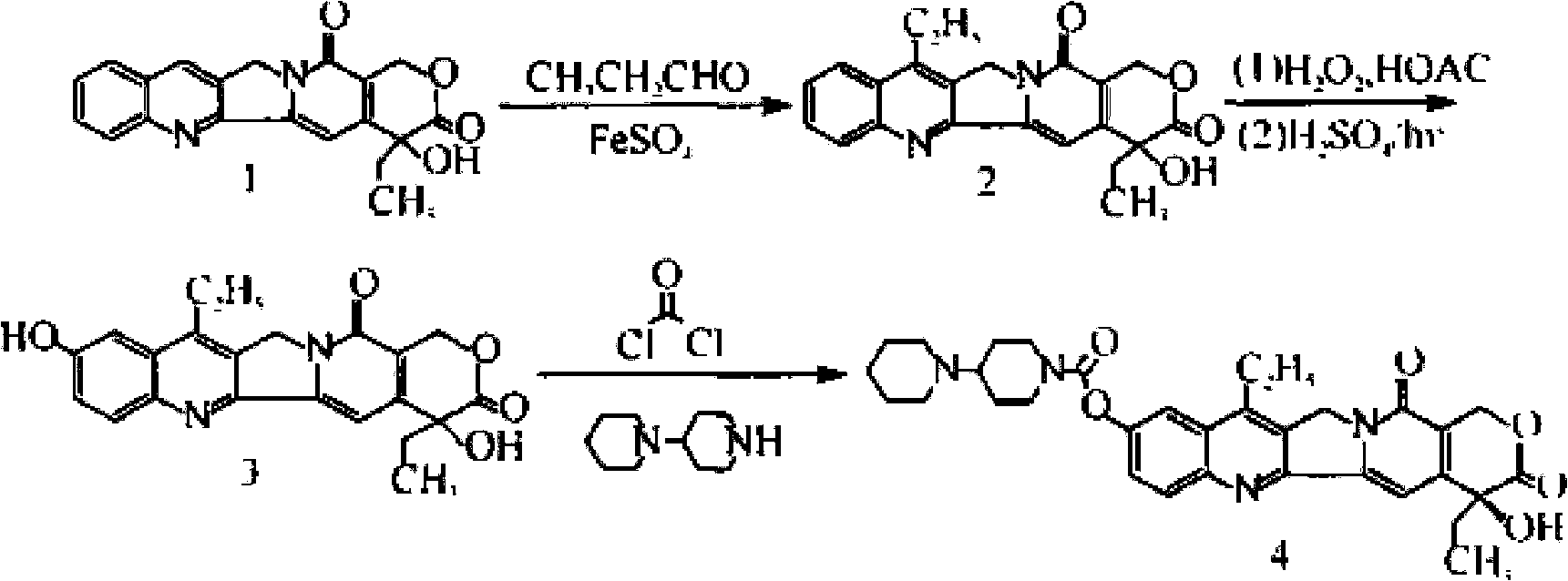
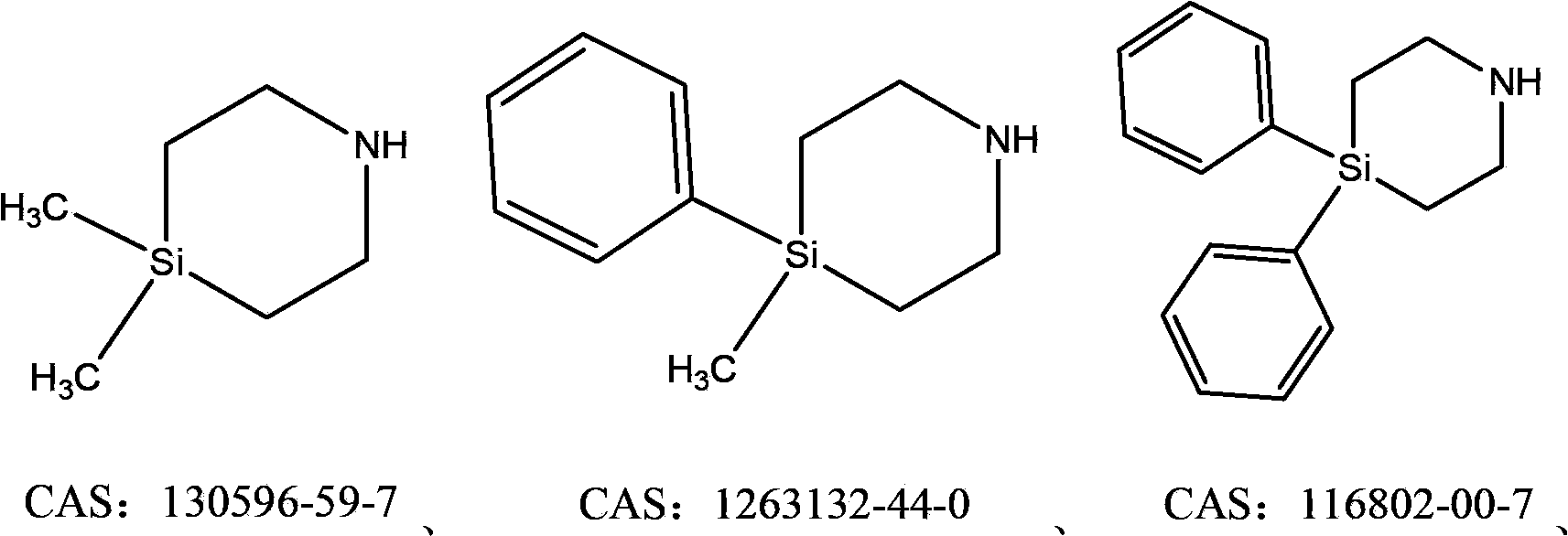
Sila piperidine derivative, preparation method and use thereof
A technology of silapiperidine and derivatives, which is applied in the field of drug synthesis, and can solve the problems of complex synthetic process routes, difficulty in large-scale production, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: Preparation of 4-methyl-4-(4-fluorophenyl)-[1,4]silapiperidine (No. 1)
[0106] Step 1, Preparation of Methyl-4-Fluorophenyldivinylsilane (Intermediate 2)
[0107] Take the starting material 1 methyl-(4-fluorophenyl)-dichlorosilane (318g, 1.52mol) and dissolve it in 5000ml of anhydrous ether, add vinylmagnesium chloride (2000ml, 1.6M) dropwise in an ice-water bath, and keep the temperature at 0-20°C. After the dropwise addition was completed, the temperature was raised naturally for 8-16 hours. After the reaction was completed, quenched with water at 0°C, filtered off the solid, and distilled off the solvent to obtain a light brown liquid, namely methyl-(4-fluorophenyl)-divinylsilane (intermediate product 2), with a yield of 82-89% %.
[0108] 1H NMR (400MHz, CDCl 3 ), δ(ppm)=7.45(d,J=8.4,2H),6.94(m,2H),6.32(d,J=16,2H),6.14(d,J=16,2H),5.77(d ,J=16,2H),0.43(s,3H).MS-ESI(M+H + )=193.10.
[0109] Step 2, the preparation of methyl-(4-fluorophenyl)-bis(2-br...
Embodiment 2
[0118] Example 2: Preparation of 4-methyl-4-(4-trifluoromethylphenyl)-[1,4]silapiperidine (No. 2)
[0119] Step 1, preparation of methyl-(4-trifluoromethylphenyl)-divinylsilane (intermediate 2)
[0120] Dissolve the starting material 1 methyl-(4-trifluoromethylphenyl)-dichlorosilane (389g, 1.50mol) in 5000ml of anhydrous ether, and add vinylmagnesium chloride (2000ml, 1.6M) dropwise under an ice-water bath , keep the temperature at 0-20°C. After the dropwise addition was completed, the temperature was raised naturally for 8-16 hours. After the reaction was completed, water was added to quench at 0°C, the solid was filtered off, and the solvent was distilled off to obtain a light brown liquid, which was methyl-(4-trifluoromethylphenyl)-divinylsilane (intermediate product 2), yield 79-84%.
[0121] 1H NMR (400MHz, CDCl 3 ), δ(ppm)=7.49(d,J=8.4,2H),7.46(d,J=8.4,2H),6.33(d,J=16,2H),6.15(d,J=16,2H) ,5.77(d,J=16,2H),0.44(s,3H).MS-ESI(M+H + )=243.08.
[0122] Step 2, the prepa...
Embodiment 3
[0131] Example 3: Preparation of 4-methyl-4-(4-methoxyphenyl)-[1,4]silapiperidine (No. 3)
[0132] Step 1, preparation of methyl-(4-methoxyphenyl)-divinylsilane (intermediate 2)
[0133] Take the starting material 1 methyl-(4-methoxyphenyl)-dichlorosilane (334g, 1.51mol) and dissolve it in 5000ml of anhydrous ether, add vinylmagnesium chloride (2000ml, 1.6M) dropwise under ice-water bath, Keep the temperature 0-20°C. After the dropwise addition was completed, the temperature was raised naturally for 8-16 hours. After the reaction was completed, water was added to quench at 0°C, the solid was filtered off, and the solvent was distilled off to obtain a light brown liquid, namely methyl-(4-methoxyphenyl)-divinylsilane (intermediate product 2), with a yield of 82 -85%.
[0134] 1H NMR (400MHz, CDCl 3 )δ(ppm)=7.39(d,J=8.4,2H),6.75(d,J=8.4,2H),6.32(d,J=16,2H),6.13(d,J=16,2H), 5.76(d,J=16,2H),0.44(s,3H).MS-ESI(M+H + )=205.07.
[0135] Step 2, the preparation of methyl-(4-metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com