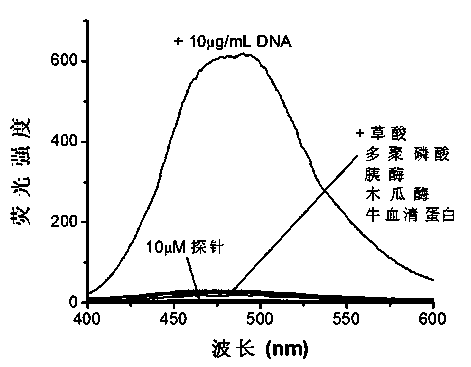
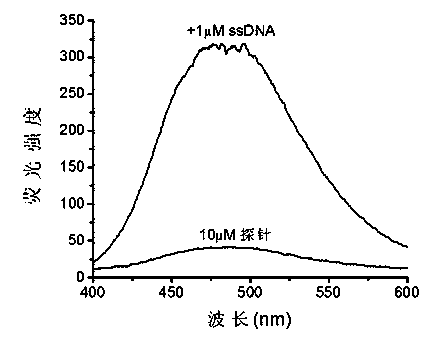
Nucleic acid fluorescence probe and preparation method thereof
A compound and alkyl technology, applied in the field of nucleic acid fluorescent probes and their preparation, can solve the problems of high cost, strong carcinogenicity and complex operation of nucleic acid probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 probe 4 and 5
[0035]
[0036] Mix 0.82g of a1 (synthesized by reference Chem.Commun.2006, 3705–3707), 0.24g of sodium azide and 15mL of dimethyl sulfoxide, stir at 80°C for 3 hours, pour into water, and the precipitated insoluble Purified by column chromatography to obtain 0.7 g of light yellow oil, ie intermediate b, with a yield of 98%. 1 H NMR (300MHz, CDCl 3 )δ[ppm]:7.15-7.0(m,10H),7.00-6.90(m,4H),6.69-6.62(m,4H),4.11-4.04(m,4H),3.59-3.52(m,4H) .
[0037]Dissolve 0.5g of b and 1.07g of triphenylphosphine in 120mL of tetrahydrofuran, add 20mL of water, stir overnight at 60°C, distill off the solvent under reduced pressure, and use chloroform-methanol gradient eluent column chromatography to obtain probe 5 in sequence. and 4 were concentrated under reduced pressure to obtain 0.19 g and 0.185 g of white solids, and the yields were 44% and 41%, respectively. Probe 4: ESI-MS m / z[M+H] + 451; Probe 5: ESI-MS m / z [M+H] + 451.
Embodiment 2
[0038] The synthesis of embodiment 2 probe 8
[0039]
[0040] Using a2 (synthesized in reference Chem.Eur.J.2008,14,6428–6437) as raw material, according to the synthesis method of probe 4 and probe 5, the amount of sodium azide and triphenylphosphine was doubled, and the Probe 8 was obtained as a white solid. ESI-MS m / z[M+H] + 569.
Embodiment 3
[0041] Synthesis of Example 3 Probes 11 and 12
[0042]
[0043] Using a3 (synthesized by reference Org. Lett. 2008, 10, 4581–4584) as raw material, according to the synthesis method of probe 4 and probe 5, probes 11 and 12 can be obtained as white or yellow solids. Probe 11: ESI-MSm / z[M+H] + 507; Probe 12: ESI-MS m / z [M+H] + 507.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com