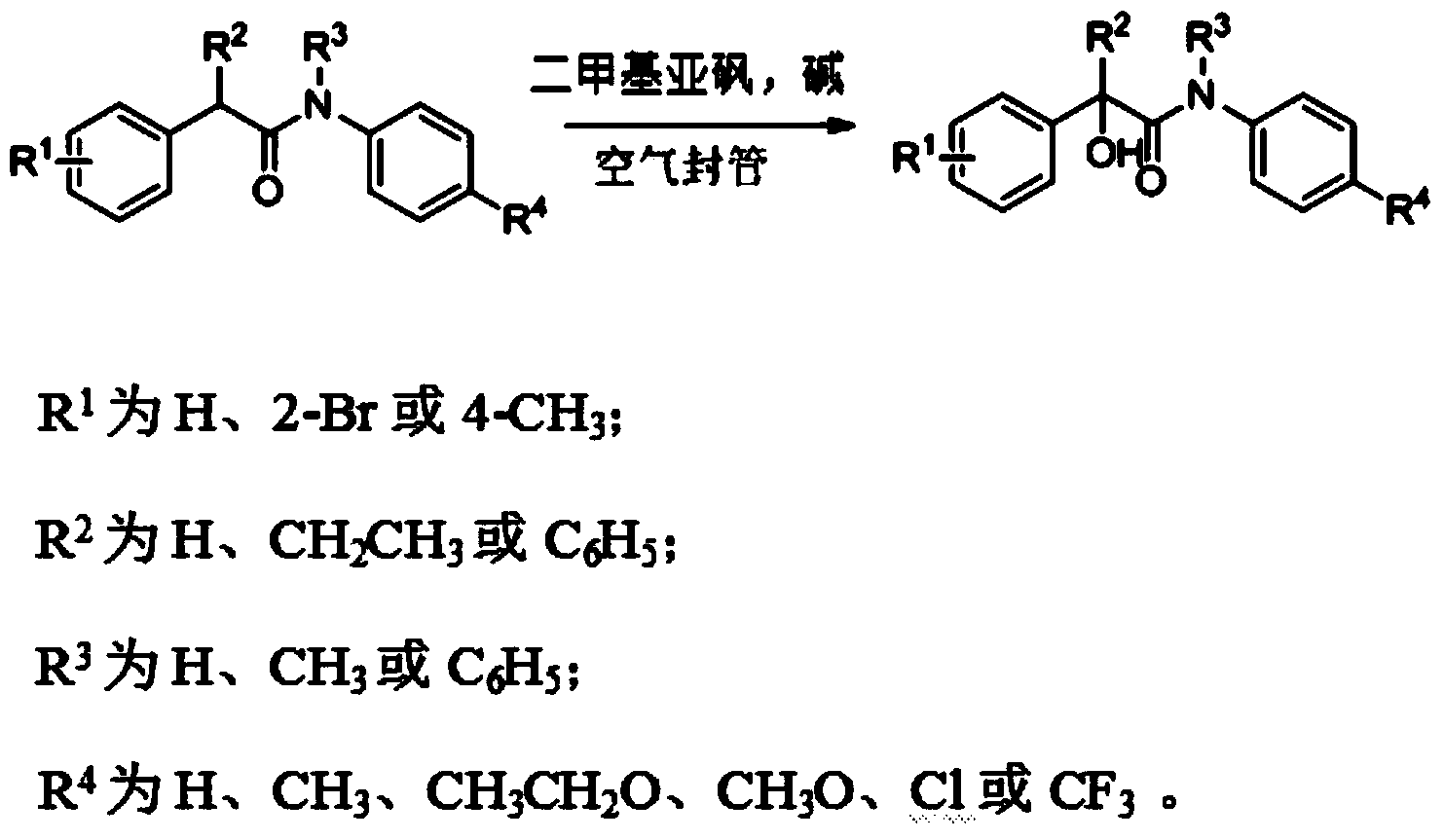
Method for synthesizing alpha-hydroxy amide compound
A synthetic method, the technology of hydroxyamide, applied in the field of organic chemical synthesis, can solve the problems of difficult control, many reaction steps, low temperature, etc., and achieve the effect of easy operation control, high reaction efficiency and appropriate temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
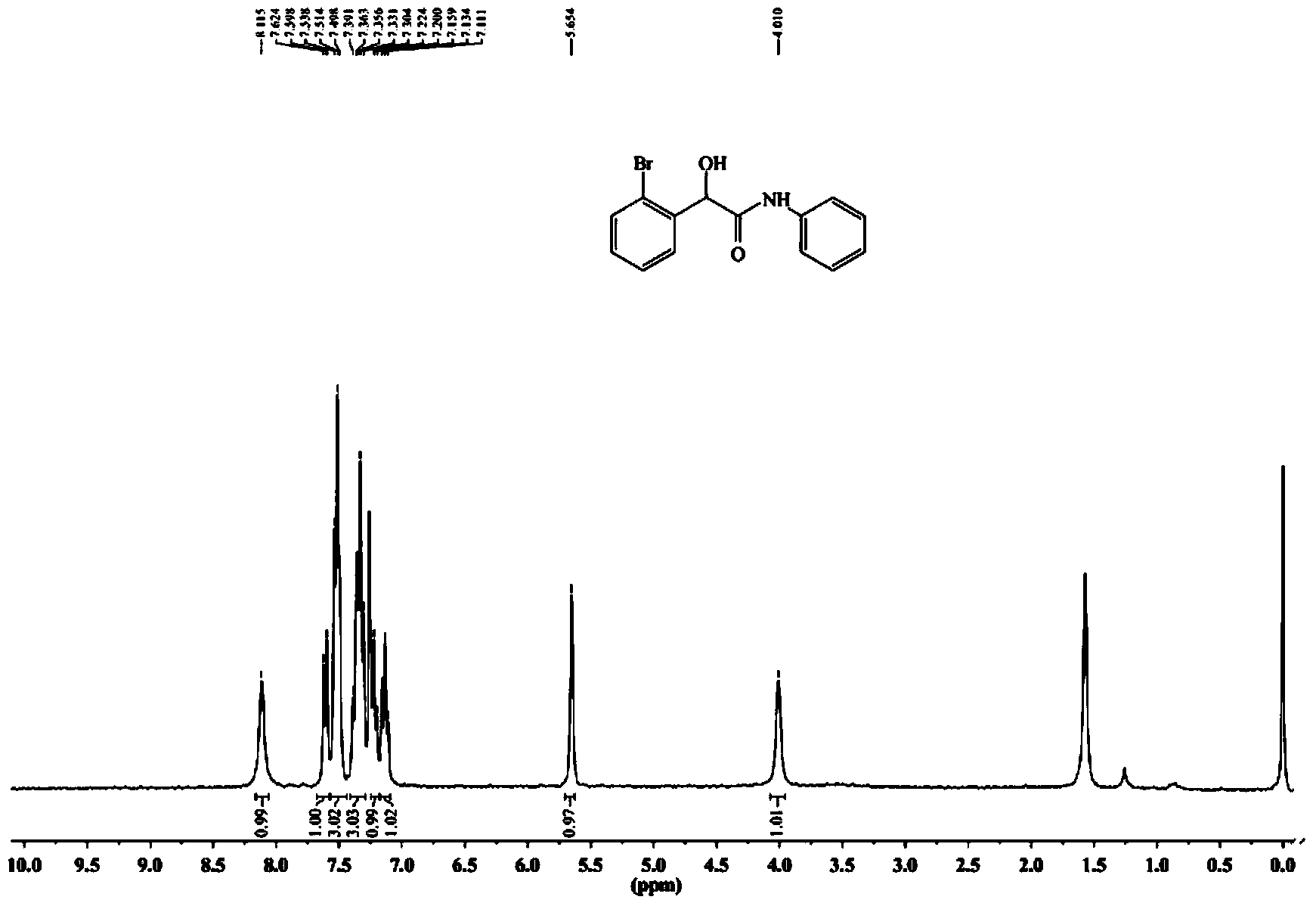
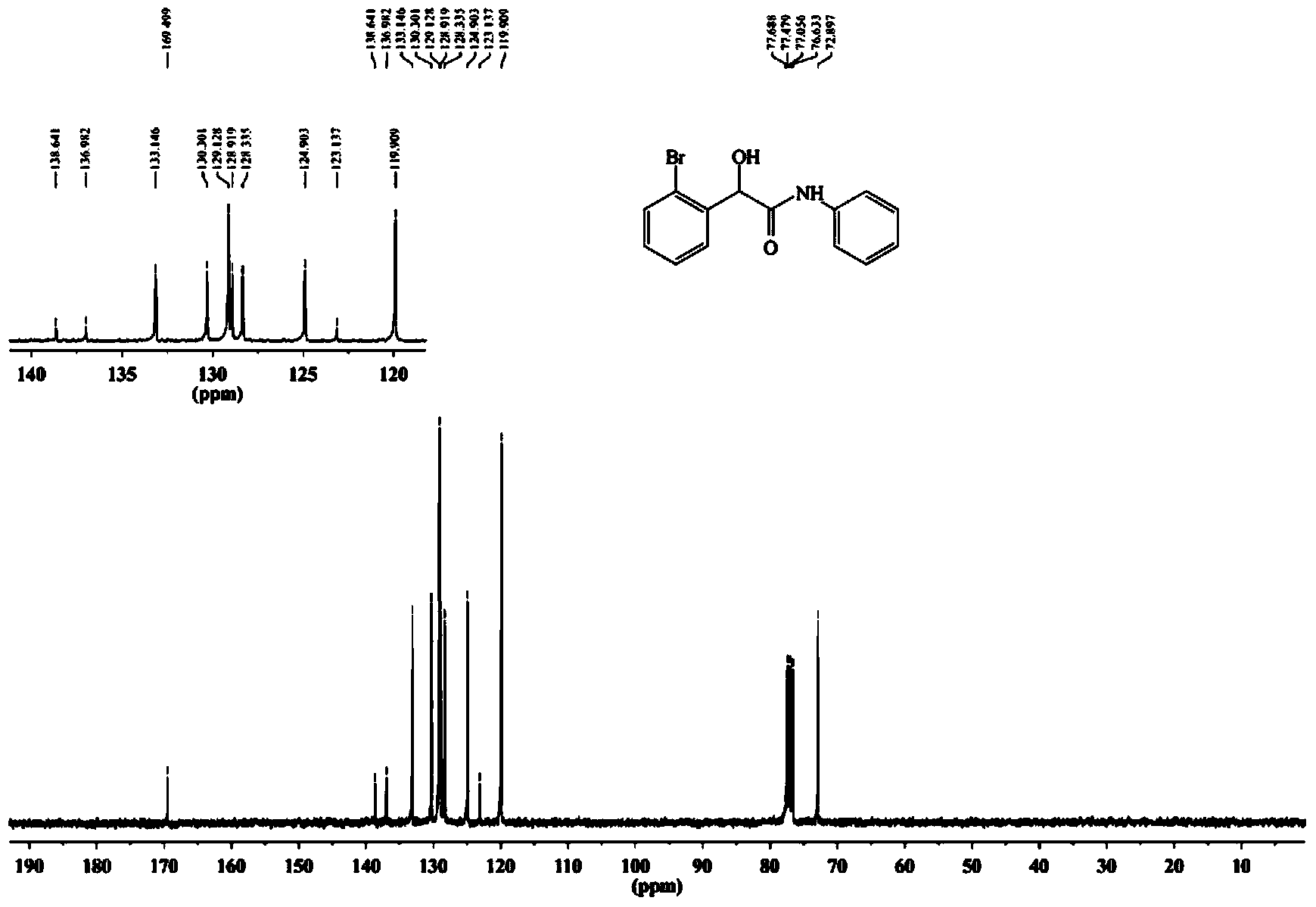
[0075] The synthetic method of 2-(2-bromophenyl)-2-hydroxyl-N-phenylacetamide specifically comprises the following steps:
[0076] (1) Add 0.3 grams of 2-(2-bromophenyl)-N-phenylacetamide, 0.15 grams of potassium hydroxide, and 10 milliliters of dimethyl sulfoxide to a 50-ml two-necked flask, and in air atmosphere Seal the tube and stir it magnetically at 50°C for 24 hours;
[0077] (2) After the reaction, cool to room temperature, add saturated brine to wash, extract 3 times with ethyl acetate, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain crude product;
[0078] (3) The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 4:1) to obtain a white solid, namely 2-(2-bromophenyl)-2-hydroxy -N-phenylacetamide, yield 81%, melting point: 140-141°C.
[0079] The resul...
Embodiment 2
[0084] The synthetic method of 2-(2-bromophenyl)-2-hydroxyl-N-(4-methylphenyl)acetamide specifically comprises the following steps:
[0085] (1) Add 3.2 grams of 2-(2-bromophenyl)-N-(4-methylphenyl)acetamide, 1.6 grams of potassium hydroxide, and 100 milliliters of dimethyl Sulfone, sealed in air atmosphere, and magnetically stirred at 50°C for 24 hours;
[0086] (2) After the reaction, cool to room temperature, add saturated brine to wash, extract 4 times with ethyl acetate, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain crude product;
[0087] (3) The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 4:1) to obtain a white solid, namely 2-(2-bromophenyl)-2-hydroxy -N-(4-methylphenyl)acetamide, the yield is 84%, melting point: 148-149°C.
[0088] The resultin...
Embodiment 3
[0093] The synthetic method of 2-(2-bromophenyl)-2-hydroxyl-N-(4-ethoxyphenyl) acetamide specifically comprises the following steps:
[0094] (1) Add 3.5 grams of 2-(2-bromophenyl)-N-(4-ethoxyphenyl)acetamide, 1.5 grams of potassium hydroxide, and 80 milliliters of dimethylmethylene to a 250-ml two-necked flask. Sulfone, sealed in air atmosphere, and magnetically stirred at 50°C for 24 hours;
[0095] (2) After the reaction, cool to room temperature, add saturated brine to wash, extract 5 times with ethyl acetate, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain crude product;
[0096] (3) The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 5:1) to obtain a white solid, namely 2-(2-bromophenyl)-2-hydroxy -N-(4-ethoxyphenyl)acetamide, yield 80%, melting point: 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com