A kind of preparation method of erythrostructure methoxamine hydrochloride
A technology of methoxamine hydrochloride and its structural formula, which is applied in the field of preparation of methoxamine hydrochloride with erythromorphic structure, can solve problems such as adverse effects on the human body and the environment, difficulty in obtaining raw materials, unfavorable industrial production, etc., and achieve excellent product quality and increased hydrogenation speed , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
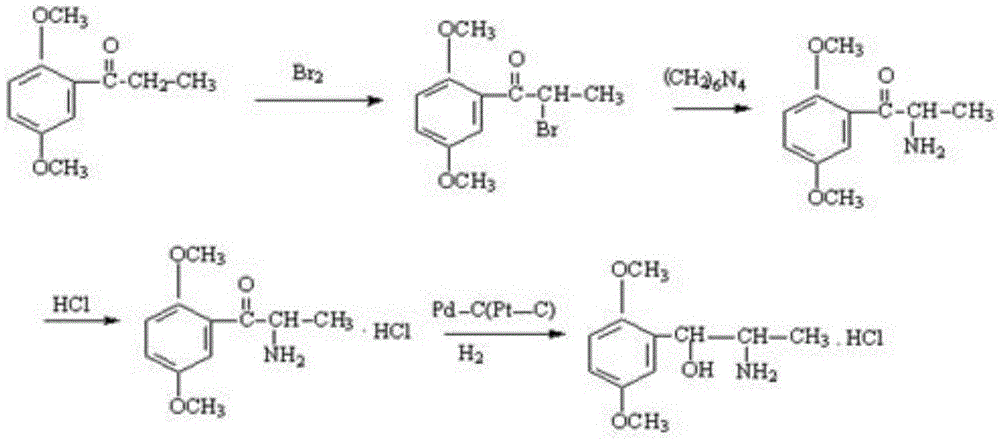
[0027] The invention discloses a preparation method of methoxamine hydrochloride with an erythrogenic structure, the method comprising the following steps:
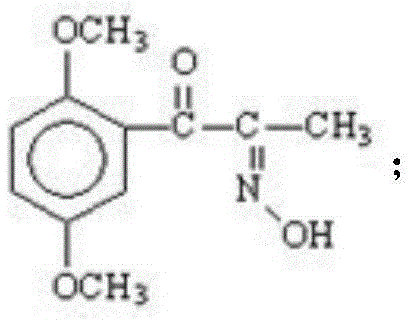
[0028] (1) The initial raw material 2,5-dimethylpropiophenone is dissolved in an organic solvent, the organic solvent is ethyl acetate, butyl acetate, chloroform, dichloromethane, carbon tetrachloride or ether, and it is passed through to dry Hydrogen chloride gas, until the hydrogen chloride saturation state in the solvent is better, then add n-butyl nitrite solution dropwise for oximation reaction, keep the temperature at 5-30°C during the dropwise addition of n-butyl nitrite solution, after the dropwise addition is complete, React to the end at a temperature of 0-30°C to obtain intermediate I, the structural formula of intermediate I is:
[0029]
[0030] (2) Under acidic conditions, using methanol solution as a solvent and palladium carbon as a catalyst, hydrogen is used to reduce the oxime group in intermediate I....
Embodiment 1
[0039] (1) Preparation of 2,5-dimethoxy-α-isonitrosopropiophenone (intermediate I):
[0040] In a 10L three-necked flask, add 3500ml of anhydrous diethyl ether, stir, cool in an ice-water bath to below 20°C, pass in dry hydrogen chloride gas, then add 700g of 2,5-dimethoxypropiophenone, until the hydrogen chloride gas in the reaction solution is saturated, Slowly add 525ml of n-butyl nitrite dropwise to the reaction solution, continue to feed hydrogen chloride gas until the solution is saturated, remove the ice-water bath, stop the reaction completely, and filter with suction, rinse the filter cake with a small amount of ether to obtain a light yellow solid 2, 603 g of 5-dimethoxy-α-isonitrosopropiophenone (intermediate I) was calculated, and the yield was 86.14%.
[0041] (2) Preparation of 2,5-dimethoxy-α-aminopropiophenone hydrochloride (intermediate II):
[0042] Add 244g of 10% palladium carbon, 600g of intermediate I, 3.05L of methanolic hydrogen chloride solution, and ...
Embodiment 2
[0046] (1) Preparation of 2,5-dimethoxy-α-isonitrosopropiophenone (intermediate I):
[0047] In a 10L three-necked flask, add 3500ml of carbon tetrachloride, pass through dry hydrogen chloride gas, then add 500g of 2,5-dimethoxypropiophenone, until the hydrogen chloride gas in the reaction solution is saturated, slowly add 400ml of n-butyl nitrate (about 1h), and maintain the temperature at 30 ° C, dropwise, stop feeding hydrogen chloride gas and continue to stir at room temperature for 6 hours, stop stirring, place at room temperature for 14 hours, suction filter, filter cake with a small amount of Rinse with ether to obtain 2,5-dimethoxy-α-isonitrosopropiophenone (Intermediate I) 455 as a pale yellow solid, and the calculated yield is 91%.
[0048] (2) Preparation of 2,5-dimethoxy-α-aminopropiophenone hydrochloride (intermediate II):
[0049] Add 279g of 10% Raney nickel, 450g of intermediate I, 3.05L of hydrogen chloride methanol solution, and 3.05L of methanol into a 10L th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com