Method for realizing stable electrochemical activity of polyaniline in neutral medium
A neutral medium, electrochemical technology, applied in the field of electrochemistry, can solve the problems of poor cycle stability and inability to meet the needs of polyaniline, and achieve the effects of stabilizing electrochemical activity, increasing surface area, and bonding tightly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] First, a tungsten foil with a purity of 99.9%, a length of 40 mm, a width of 10 mm, and a thickness of 100 μm was taken and soaked in 2 wt% NaOH solution for 2 min to remove the natural oxide film on the surface of the tungsten foil. Then ultrasonically clean with ethanol and deionized water for 10 min respectively to remove oil-soluble and water-soluble impurities on the surface of tungsten foil. With tungsten foil as anode and graphite rod as cathode, 0.1 mol L -1 The perchloric acid aqueous solution was used as the electrolyte for constant voltage anodic oxidation, the oxidation voltage was 50V, the electrolyte temperature was 30°C, and the oxidation time was 10 min.
[0022] Anneal the prepared tungsten oxide in air, raise the temperature to 450°C and keep it for 3 hours, and finally cool down to room temperature naturally, and the heating rate is 20°C / min.
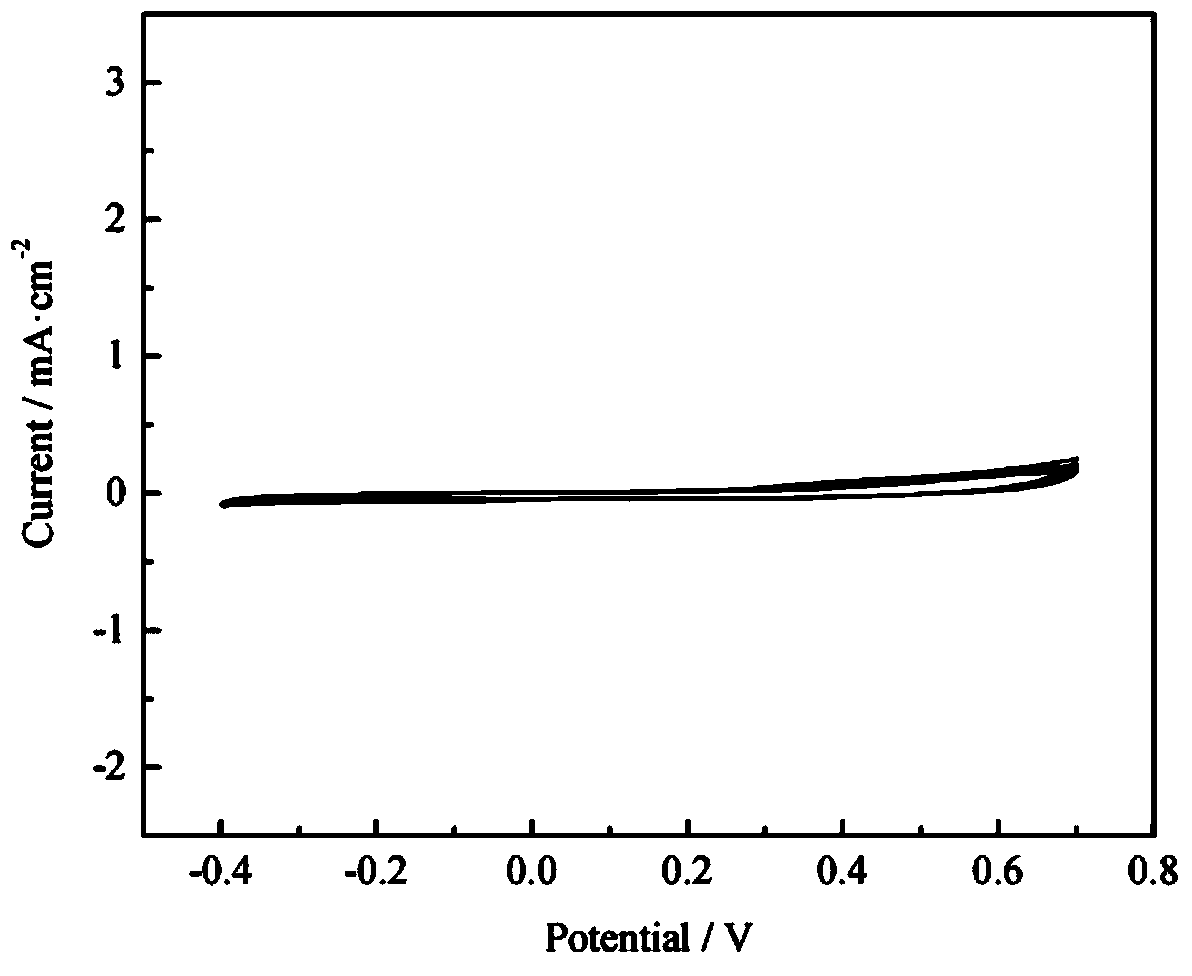
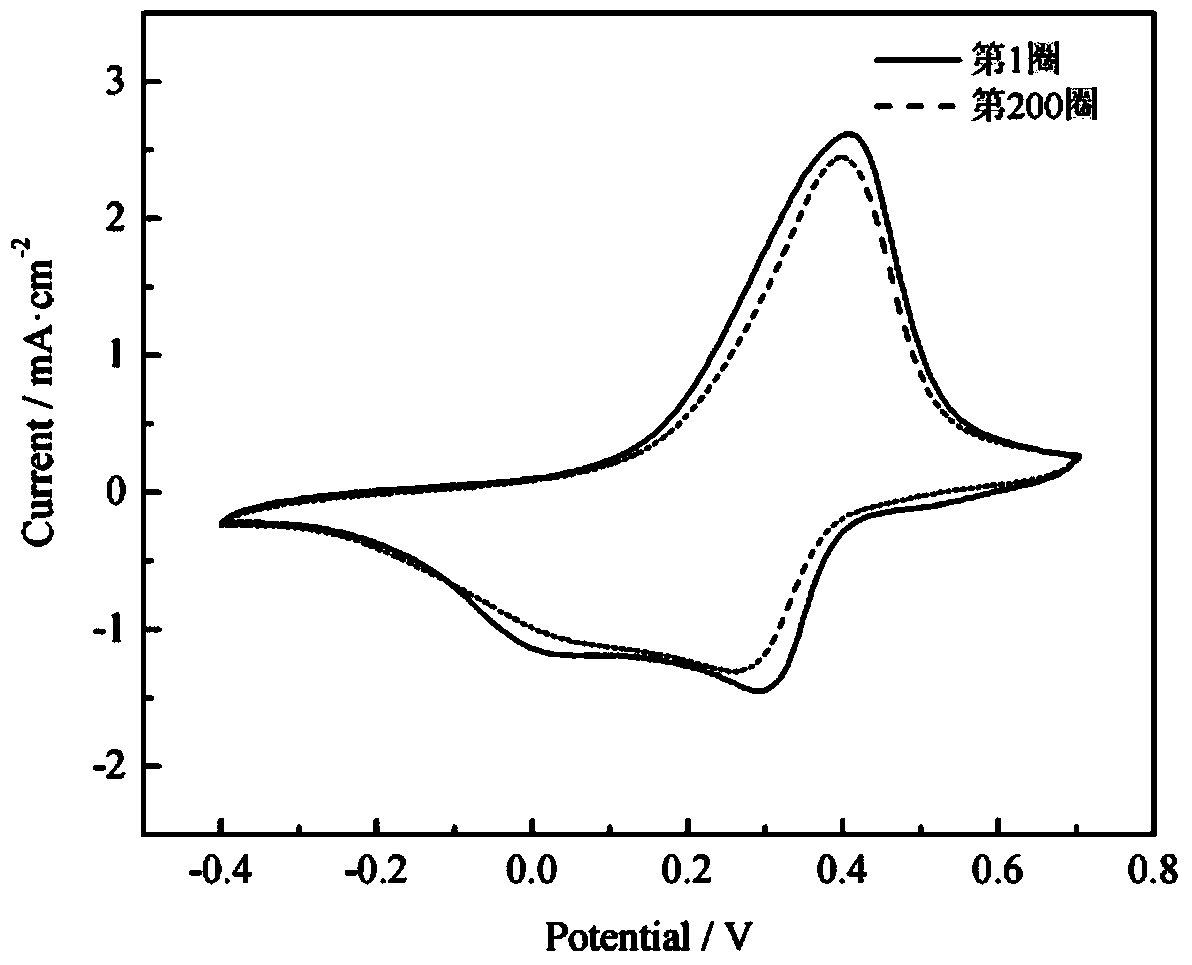
[0023] The annealed tungsten oxide was used as the working electrode, the graphite rod was used as the coun...
Embodiment 2
[0026] The purity, size, pretreatment and anodizing process of the tungsten foil are the same as in Example 1, and the anodizing time is changed to 5 min.
[0027] The annealing process of tungsten oxide, the aniline polymerization conditions and the cyclic voltammetry test method of the polyaniline film are the same as in Example 1. The results show that after 200 cycles of continuous scanning, the electrode still has stable electrochemical activity, and the peak current and peak position remain basically unchanged compared with the first cycle.
Embodiment 3
[0029] The purity, size, pretreatment and anodizing process of the tungsten foil are the same as in Example 1, and the anodizing voltage is changed to 55V, and the oxidation time is set to 5 min.
[0030] The annealing process of tungsten oxide, the aniline polymerization conditions and the cyclic voltammetry test method of the polyaniline film are the same as in Example 1. The results show that after 200 cycles of continuous scanning, the electrode still has stable electrochemical activity, and the peak current and peak position remain basically unchanged compared with the first cycle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com